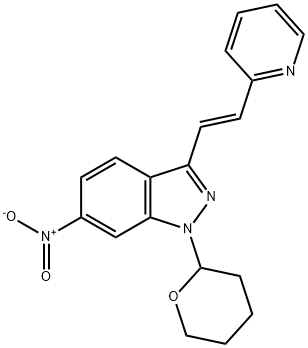
(E)-6-Nitro-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole synthesis
- Product Name:(E)-6-Nitro-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole
- CAS Number:886230-75-7
- Molecular formula:C19H18N4O3
- Molecular Weight:350.37
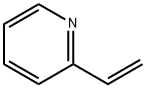
100-69-6
211 suppliers
$14.00/1mL
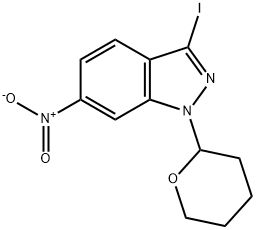
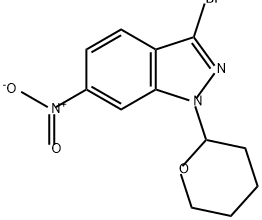
886230-74-6
54 suppliers
$359.04/1g
![(E)-6-Nitro-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole](/CAS2/GIF/886230-75-7.gif)
886230-75-7
80 suppliers
inquiry
Yield:886230-75-7 96%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;tris-(o-tolyl)phosphine in N,N-dimethyl-formamide at 100; for 12 h;
Steps:
1c
Step Ic. (E)-6-nitro-3-(2-(pyridin-2-yl)vinyl)-l-(tetrahydro-2H-pyran-2-yl)-lH- indazole (Compound 104) Compound 103 (24.4g, 65.4 mmol) was added to a solution of 2-vinyl pyridine(9.82g, 93.4 mmol), JV,jV-diisopropylethylamine (16.2g, 125 mmol) and tri-o- tolylphosphine (1.72g, 5.65 mmol) in DMF (163 g) . PdCl2 (0.38g, 2.1 mmol) was added and the mixture was agitated for 12 h at 100 0C (until the reaction was completed by ηPLC). The mixture was then cooled to 45 0C and isopropanol (80 g) was added. The mixture was agitated for 30 min at 45 0C, diluted with water (400 mL), and the mixture was agitated at 25 0C for 1 h. The resulting slurry was filtered, rinsed with water (25 mL), and the solids were combined with isopropanol (100 g). The mixture was agitated for 30 min at 55 0C, then for 30 min at 10 0C, filtered, and the solids were washed with cold isopropanol (2x10 mL). The solids were dried in a vacuum oven for 12 h (5O0C and 25 mmηg) to provide compound 104 (22g, 96% yield): LCMS: 351 [M+l].
References:
WO2009/36066,2009,A1 Location in patent:Page/Page column 52
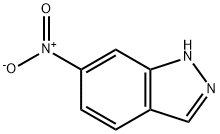
7597-18-4
303 suppliers
$17.00/25g
![(E)-6-Nitro-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole](/CAS2/GIF/886230-75-7.gif)
886230-75-7
80 suppliers
inquiry
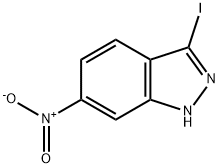
70315-70-7
197 suppliers
$10.00/250mg
![(E)-6-Nitro-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole](/CAS2/GIF/886230-75-7.gif)
886230-75-7
80 suppliers
inquiry
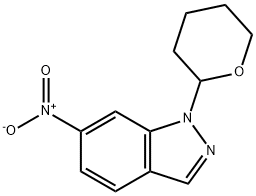
150187-67-0
11 suppliers
inquiry
![(E)-6-Nitro-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole](/CAS2/GIF/886230-75-7.gif)
886230-75-7
80 suppliers
inquiry