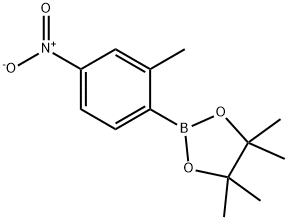
4,4,5,5-TETRAMETHYL-2-(2-METHYL-4-NITROPHENYL)-1,3,2-DIOXABOROLANE synthesis
- Product Name:4,4,5,5-TETRAMETHYL-2-(2-METHYL-4-NITROPHENYL)-1,3,2-DIOXABOROLANE
- CAS Number:883715-40-0
- Molecular formula:C13H18BNO4
- Molecular Weight:263.1
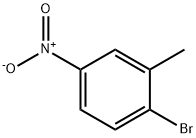
7149-70-4
183 suppliers
$9.00/1g
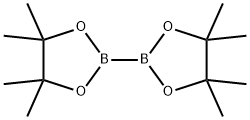
73183-34-3
579 suppliers
$6.00/5g

883715-40-0
41 suppliers
$194.00/1g
Yield:883715-40-0 90%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 100; for 16 h;Inert atmosphere;
Steps:
182.1 Step-1: Synthesis of 4,4,5,5-tetramethyl-2-(2-methyl-4-nitrophenyl)-1,3,2- dioxaborolane (182-2):
To a stirred solution of 1-bromo-2-methyl-4-nitrobenzene (182-1, 2.00 g, 9.3 mmol) in 1,4-dioxane (30 mL) under argon atmosphere were added bis(pinacolato)diboron (3.53 g, 13.95 mmol) and potassium acetate (1.82 g, 18.6 mmol). The reaction mixture was purged with argon for 10 min. followed by addition of PdCl2(dppf) (682 mg, 0.93 mmol.) and again the reaction mixture was purged with argon for 10 min. After addition completed, the reaction mixture was heated to 100 °C for 16 h. After this time, the reaction mixture was allowed to cool to room temperature, diluted with water (50 mL), extracted with EtOAc (3 × 300 mL). The filtrate was washed with water (2 × 25 mL) and brine (2 × 15 mL). The organic layer was dried over anhydrous sodium sulphate and concentrated under reduced pressure. The crude material was purified by column chromatography (silica gel, 50% ethyl acetate/hexanes to afford 4,4,5,5-tetramethyl-2-(2-methyl-4-nitrophenyl)-1,3,2-dioxaborolane (182-2, 2.20 g, yield: 90%) as a white solid. ESI (m/z) 264 [C13H18BNO4 + H]+.
References:
WO2021/41973,2021,A1 Location in patent:Paragraph 0183-0184

73183-34-3
579 suppliers
$6.00/5g
99-52-5
285 suppliers
$5.00/5G

883715-40-0
41 suppliers
$194.00/1g