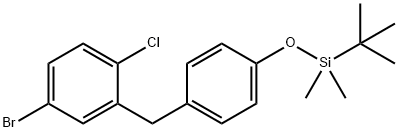
Silane,[4-[(5-broMo-2-chlorophenyl)Methyl]phenoxy](1,1-diMethylethyl)diMethyl- synthesis
- Product Name:Silane,[4-[(5-broMo-2-chlorophenyl)Methyl]phenoxy](1,1-diMethylethyl)diMethyl-
- CAS Number:864070-19-9
- Molecular formula:C19H24BrClOSi
- Molecular Weight:411.84
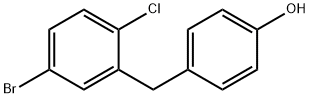
864070-18-8
177 suppliers
$35.00/1g
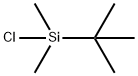
18162-48-6
673 suppliers
$9.00/5g
![Silane,[4-[(5-broMo-2-chlorophenyl)Methyl]phenoxy](1,1-diMethylethyl)diMethyl-](/CAS/20150408/GIF/864070-19-9.gif)
864070-19-9
31 suppliers
inquiry
Yield: 99%
Reaction Conditions:
with 1H-imidazole;dmap in N,N-dimethyl-formamide at 0;
Steps:
To a solution of 4-(5-bromo-2-chloro-benzyl)-phenol (9.01 g, 30.28 mmol) dissolved in Λ/, V-dimethylformamide (100 mL) and cooled to 0 degrees Celsius (ice bath) was added imidazole (4.53 g, 66.6 mmol) and 4-dimethylaminopyridine (370 mg, 3.03 mmol). tert- butylchlorodimethylsilane (6.85 g, 45.4 mmol) was added and the ice bath was removed. The reaction mixture was allowed to stir at room temperature overnight (-16 hours), water (400 mL) was added and the resulting mixture was extracted with ethyl acetate (200 mL). The aqueous layer was extracted two additional times with ethyl acetate (200 mL). The combined organic layers were washed with water (200 mL), brine (200 mL), dried over sodium sulfate, filtered and concentrated under reduced pressure. The crude material was purified by flash chromatography over silica gel using the ISCO automated chromatography unit (120g silica gel column) and eluting with a gradient of 0 to 50% ethyl acetate in heptane. 12.4g (99% yield) of product obtained as a clear oil. 1H NMR (400 MHz, CHLOROFORM-of) delta ppm 0.18 (s, 6 H), 0.97 (s, 9 H), 3.96 (s, 2 H), 6.77 (d, J=8.4 Hz, 2 H), 7.01 (d, J=8.4 Hz, 2 H), 7.17 - 7.30 (m, 3 H).
References:
PFIZER INC.;MASCITTI, Vincent WO2011/51864, 2011, A1 Location in patent:Page/Page column 28
21739-92-4
653 suppliers
$8.00/10g
![Silane,[4-[(5-broMo-2-chlorophenyl)Methyl]phenoxy](1,1-diMethylethyl)diMethyl-](/CAS/20150408/GIF/864070-19-9.gif)
864070-19-9
31 suppliers
inquiry
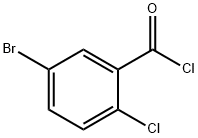
21900-52-7
83 suppliers
$12.00/250mg
![Silane,[4-[(5-broMo-2-chlorophenyl)Methyl]phenoxy](1,1-diMethylethyl)diMethyl-](/CAS/20150408/GIF/864070-19-9.gif)
864070-19-9
31 suppliers
inquiry
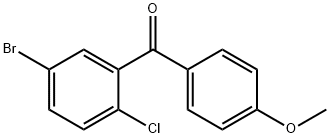
333361-49-2
36 suppliers
inquiry
![Silane,[4-[(5-broMo-2-chlorophenyl)Methyl]phenoxy](1,1-diMethylethyl)diMethyl-](/CAS/20150408/GIF/864070-19-9.gif)
864070-19-9
31 suppliers
inquiry
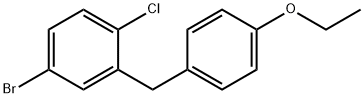
461432-23-5
503 suppliers
$30.00/1g
![Silane,[4-[(5-broMo-2-chlorophenyl)Methyl]phenoxy](1,1-diMethylethyl)diMethyl-](/CAS/20150408/GIF/864070-19-9.gif)
864070-19-9
31 suppliers
inquiry