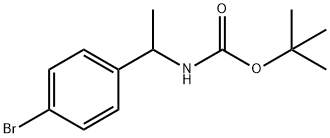
[1-(4-BROMO-PHENYL)-ETHYL]-CARBAMIC ACID TERT-BUTYL ESTER synthesis
- Product Name:[1-(4-BROMO-PHENYL)-ETHYL]-CARBAMIC ACID TERT-BUTYL ESTER
- CAS Number:850363-42-7
- Molecular formula:C13H18BrNO2
- Molecular Weight:300.19

24424-99-5
833 suppliers
$13.50/25G
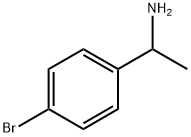
24358-62-1
149 suppliers
$9.00/1g
![[1-(4-BROMO-PHENYL)-ETHYL]-CARBAMIC ACID TERT-BUTYL ESTER](/CAS/GIF/850363-42-7.gif)
850363-42-7
53 suppliers
$45.00/50mg
Yield:850363-42-7 90%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 1 h;
Steps:
5
(Referential Example 5) Synthesis of 1-bromo-4-(1-tert-butoxycarbonylamino-ethyl)benzene (referential compound 5) Triethylamine (2.09 ml) was added to a solution of 2.00 g (10.0 mmol) of 4-(1-aminoethyl)-1-bromobenzene in 22.3 ml of dichloromethane, the mixture was cooled on an ice bath and 2.87 ml (12.0 mmol) of di-tert-butyl dicarbonate was added thereto in an argon stream with stirring. After that, temperature of the mixture was raised up to room temperature and stirring was conducted for 1 hour. After the reaction was finished, the reaction solution was poured into 200 ml of water and the mixture was extracted with 200 ml of chloroform. The organic layer was successively washed with water, a saturated aqueous solution of sodium hydrogen carbonate and a saturated aqueous solution of sodium chloride, dried over anhydrous magnesium sulfate and concentrated in vacuo. The resulting powder was washed twice with each 10 ml of hexane to give 2.71 g of the title compound as white powder (yield: 90%). Rf value: 0.55 (n-hexane: ethyl acetate = 4:1 (v/v)) Mass spectrum (FAB, m/z): 300, 302 (M+ + 1) 1H-NMR spectrum (DMSO-d6, δ ppm): 1.27 (d, J = 6.8Hz, 3H), 1.36 (brs, 9H), 4.51-4.64 (m, 1H), 7.25 (d, J = 8.3Hz, 2H), 7.35-7.45 (m, 1H), 7.49 (d, J = 8.3Hz, 2H)
References:
Ube Industries, Ltd.;SANTEN PHARMACEUTICAL CO., LTD. EP1679308, 2006, A1 Location in patent:Page/Page column 32-33

24424-99-5
833 suppliers
$13.50/25G
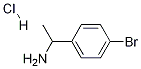
90006-14-7
26 suppliers
$94.00/5g
![[1-(4-BROMO-PHENYL)-ETHYL]-CARBAMIC ACID TERT-BUTYL ESTER](/CAS/GIF/850363-42-7.gif)
850363-42-7
53 suppliers
$45.00/50mg
110-05-4
271 suppliers
$22.00/100mL
68819-84-1
115 suppliers
$11.00/250mg
![[1-(4-BROMO-PHENYL)-ETHYL]-CARBAMIC ACID TERT-BUTYL ESTER](/CAS/GIF/850363-42-7.gif)
850363-42-7
53 suppliers
$45.00/50mg