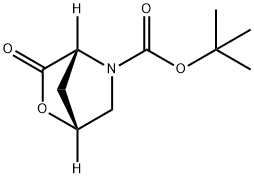
(1R,4R)-tert-Butyl 3-oxo-2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate synthesis
- Product Name:(1R,4R)-tert-Butyl 3-oxo-2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate
- CAS Number:848488-70-0
- Molecular formula:C10H15NO4
- Molecular Weight:213.23
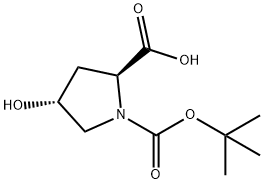
13726-69-7
434 suppliers
$5.00/1g
![(1R,4R)-tert-Butyl 3-oxo-2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate](/CAS/GIF/848488-70-0.gif)
848488-70-0
24 suppliers
$170.00/1g
Yield:848488-70-0 45%
Reaction Conditions:
Stage #1: (2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acidwith triphenylphosphine in tetrahydrofuran at 0 - 4;
Stage #2: with diethylazodicarboxylate in tetrahydrofuran at 20; for 24 h;
Steps:
A solution containing N-Boc-trans-4-hydroxyproline (71) (5 g, 21.6 mmol) and triphenylphosphine (11.8 g, 45 mmol) in anhydrous THF (150 mL) was cooled to 4° C. in an ice bath. To this solution was added DEAD (6.5 mL, 45 mmol). The reaction was allowed to stir at room temperature for 24 hours. The reaction mixture was evaporated to give a yellow oil. The crude product was purified by silica gel column chromatography to give the desired cyclic lactone (72) (2.1g, 45%):
References:
US2006/223884,2006,A1 Location in patent:Page/Page column 60
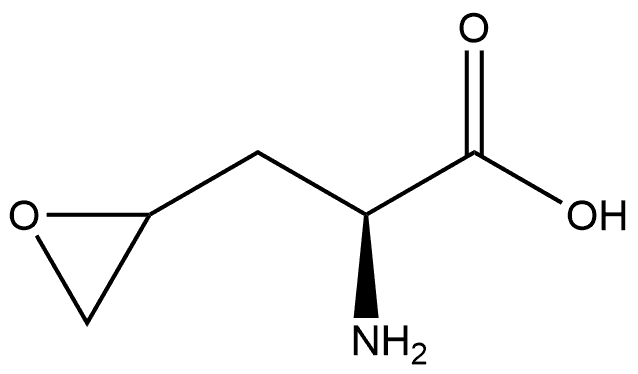
1208237-83-5
0 suppliers
inquiry
![(1R,4R)-tert-Butyl 3-oxo-2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate](/CAS/GIF/848488-70-0.gif)
848488-70-0
24 suppliers
$170.00/1g
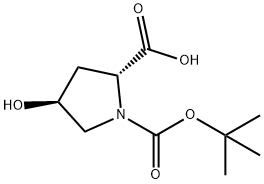
147266-92-0
173 suppliers
$60.00/100mg
![4-Pentenoic acid, 2-[[(1,1-dimethylethoxy)carbonyl]amino]-, ethyl ester](/CAS/20210111/GIF/135722-56-4.gif)
135722-56-4
0 suppliers
inquiry
![(1R,4R)-tert-Butyl 3-oxo-2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate](/CAS/GIF/848488-70-0.gif)
848488-70-0
24 suppliers
$170.00/1g

147266-92-0
173 suppliers
$60.00/100mg
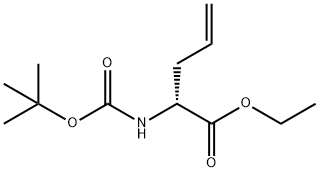
149117-85-1
1 suppliers
inquiry
![(1R,4R)-tert-Butyl 3-oxo-2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate](/CAS/GIF/848488-70-0.gif)
848488-70-0
24 suppliers
$170.00/1g

147266-92-0
173 suppliers
$60.00/100mg