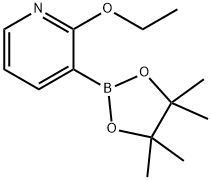
2-ETHOXY-3-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-PYRIDINE synthesis
- Product Name:2-ETHOXY-3-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-PYRIDINE
- CAS Number:848243-23-2
- Molecular formula:C13H20BNO3
- Molecular Weight:249.11
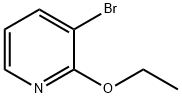
57883-25-7
104 suppliers
$72.50/250mg
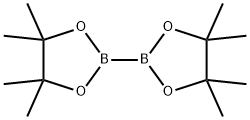
73183-34-3
578 suppliers
$6.00/5g
![2-ETHOXY-3-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-PYRIDINE](/CAS/GIF/848243-23-2.gif)
848243-23-2
61 suppliers
$45.00/100mg
Yield:848243-23-2 74%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in dimethyl sulfoxide at 95; for 6 h;Inert atmosphere;
Steps:
B.10 Preparation 10 2-ethoxy-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine
A suspension of 3-bromo-2-ethoxypyridine (Preparation 1 1 , 28.69 g, 142.0 mmol), bis(pinacolato)diboron (43.3 g, 170.5 mmol), and potassium acetate (41 .8 g, 425.9 mmol) in DMSO (100 mL) was degassed with nitrogen and [1 ,1 '- bis(diphenylphosphinoferrocene]dichloro palladium (II) (5.8 g, 7.93 mmol) was added and the reaction mixture was stirred for 6 hours at 95 °C. The reaction mixture was filtered through a pad of Arbocel which was washed with ethyl acetate (500 mL). The filtrate was concentrated in vacuo and the crude material was purified by silica gel column chromatography eluting with 50% ethyl acetate in cyclohexane to afford the title compound as a red oil (34.4g, 74%). 1H NMR (400MHz, CDCl3): δ ppm 1 .35 (s, 12H), 1 .39 (m, 3H), 4.37 (m, 2H), 6.81 (m, 1 H), 7.89 (m, 1 H), 8.19 (m, 1 H). LCMS Rt = 3.27 minutes MS m/z 250 [MH]+
References:
WO2013/93688,2013,A1 Location in patent:Page/Page column 44-45
36178-05-9
232 suppliers
$15.00/5g
![2-ETHOXY-3-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-PYRIDINE](/CAS/GIF/848243-23-2.gif)
848243-23-2
61 suppliers
$45.00/100mg