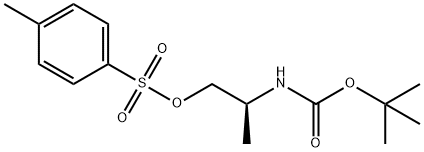
(R)-2-((tert-Butoxycarbonyl)amino)propyl 4-methylbenzenesulfonate synthesis
- Product Name:(R)-2-((tert-Butoxycarbonyl)amino)propyl 4-methylbenzenesulfonate
- CAS Number:84765-24-2
- Molecular formula:C15H23NO5S
- Molecular Weight:329.41
Yield:84765-24-2 90.5%
Reaction Conditions:
with dmap;triethylamine in dichloromethane at 5 - 20; for 12 h;
Steps:
2 Example 2 Preparation of (S)-2-((tert-butoxycarbonyl)amino)propyl 4-methylbenzenesulfonate (compound 3a)
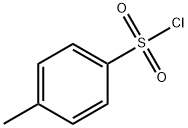
Compound 2 (100.0 g), 0.8 L of dichloromethane, 3.4 g of 4-dimethylaminopyridine, and 86.6 g of triethylamine were successively added to the reaction flask. A solution of p-toluenesulfonyl chloride (114.6 g) in dichloromethane (0.2 L) was added dropwise to the reaction solution under temperature control at 5°C; after the dropwise addition, the temperature was raised to 20°C and kept for 12 hours. The reaction solution was cooled to 5°C and kept stirring for 30 minutes, filtered, the filtrate was washed successively with 10% aqueous citric acid solution and saturated aqueous sodium bicarbonate solution, the organic phase was dried with anhydrous sodium sulfate and concentrated to dryness under reduced pressure to obtain compound 3a, It was 170.1 g of off-white solid, yield: 90.5%, HPLC: 92.2%.
References:
CN114230490,2022,A Location in patent:Paragraph 0143-0147

24424-99-5
833 suppliers
$13.50/25G

84765-24-2
11 suppliers
inquiry
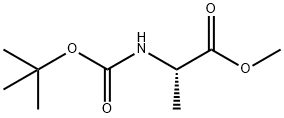
28875-17-4
145 suppliers
$6.00/5g

84765-24-2
11 suppliers
inquiry
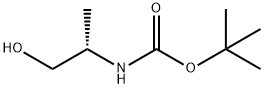
79069-13-9
379 suppliers
$5.00/1g

84765-24-2
11 suppliers
inquiry