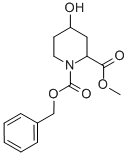
4-HYDROXY-PIPERIDINE-1,2-DICARBOXYLIC ACID 1-BENZYL ESTER 2-METHYL ESTER synthesis
- Product Name:4-HYDROXY-PIPERIDINE-1,2-DICARBOXYLIC ACID 1-BENZYL ESTER 2-METHYL ESTER
- CAS Number:847029-99-6
- Molecular formula:C15H19NO5
- Molecular Weight:293.32
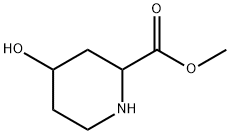
144913-66-6
33 suppliers
$190.00/1g
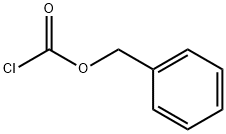
501-53-1
395 suppliers
$10.00/1g

847029-99-6
2 suppliers
inquiry
Yield:-
Reaction Conditions:
with triethylamine in dichloromethane at 5 - 20;
Steps:
Formation of 1-benzyl 2-methyl 4-hydroxypiperidine-1,2-dicarboxylate (13b)
Formation of 1-benzyl 2-methyl 4-hydroxypiperidine-1,2-dicarboxylate (13b) (1766) To a cold (5° C.) solution of methyl 4-hydroxypiperidine-2-carboxylate, 13a, (5.17 g, 32.48 mmol) and triethylamine (6.00 mL, 43.05 mmol) in CH2Cl2 (135 mL) was added dropwise benzyl chloroformate (6.20 mL, 43.43 mmol) over 10 min. The resulting solution was stirred at 5° C. for 1 hour and then allowed to warm to room temperature. The reaction mixture was diluted with water and the layers were separated. The aqueous was re-extracted with CH2Cl2 and the combined organics were dried over MgSO4, filtered and evaporated to dryness. The crude was passed through a plug of silica gel, eluting with 30-80% EtOAc/Hexanes to afford the desired product, 13b. (1767) 1H NMR (300 MHz, CDCl3) δ 7.36-7.33 (m, 5H), 5.17 (s, 2H), 4.89-4.78 (m, 1H), 4.18-4.09 (m, 1H), 3.96 (s, 1H), 3.76-3.70 (m, 3H), 3.53-3.41 (m, 2H), 2.44 (s, 1H), 1.96-1.91 (m, 1H) and 1.71 (s, 2H) ppm.
References:
US9345708,2016,B2 Location in patent:Page/Page column 252