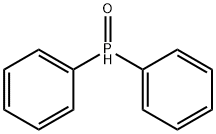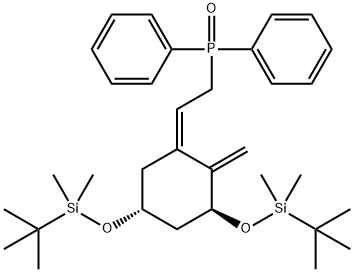
PHOSPHINE OXIDE, [2-[3,5-BIS [[(1,1-DIMETHYLETHYL) DIMETHYLSILY]OXY]-2-METHYLENECYCLOHEXYLIDENE]ETHYL]DIPHENYL-,[3S-(1Z,3A,5B0)] synthesis
- Product Name:PHOSPHINE OXIDE, [2-[3,5-BIS [[(1,1-DIMETHYLETHYL) DIMETHYLSILY]OXY]-2-METHYLENECYCLOHEXYLIDENE]ETHYL]DIPHENYL-,[3S-(1Z,3A,5B0)]
- CAS Number:81522-68-1
- Molecular formula:C33H51O3PSi2
- Molecular Weight:582.9
Yield:81522-68-1 50%
Reaction Conditions:
Stage #1: Diphenylphosphine oxidewith potassium hydroxide;tetrabutylammomium bromide in tert-butyl methyl ether;water at 20; for 0.25 h;
Stage #2: <3S-(1Z,3α,5β)>-2-<3,5-bis<<(1,1-dimethylethyl)dimethylsilyl>oxy>-2-methylenecyclohexylidene>-1-chloroethane in tert-butyl methyl ether;water at 20; for 2.08333 - 2.16667 h;
Steps:
1 Preparation of 3S-(3α,5β,Z)-2-2-2-methylene-bis(1,1-dimethylethyl)dimethyl-silyl-oxy-cyclohexylidene-ethyl-diphenyl phosphine oxide
Example 1 Preparation of 3S-(3α,5β,Z)-2-2-2-methylene-bis(1,1-dimethylethyl)dimethyl-silyl-oxy-cyclohexylidene-ethyl-diphenyl phosphine oxide Potassium hydroxide (1.0 g, 17.97 mmole) was dissolved in water (0.5 mL) and was then added to a mixture of tetra-n-butylammonium bromide (TBAB) (1.16 g, 3.59 mmole) as phase transfer catalyst and diphenyl phosphine oxide (2.9 g, 14.37 mmole, prepared from chloro-diphenyl phosphine) in methyl t-butyl ether (MTBE) (40 mL) at room temperature. After stirring for 15 minutes, a solution of the chloro-compound (Z)-(1 S,5R)-1,5-bis-(tert-butyl-dimethyl-silanyloxy)-3-(2-chloro-ethylidene)-2-methylene-cyclohexane (5.0 g, 11.98 mmole) in MTBE (15 mL) was added drop-wise (during 5-10 min.). The reaction mixture was then stirred for 2 hours at room temperature. Thin Layer Chromatography (TLC) showed only a small amount of starting material (5-10%). The reaction product was diluted with MTBE (50 mL) and water (25 mL). The layers were separated and the organic layer was washed with water (25 mL) and brine (25 mL), dried with anhydrous Na2SO4, filtered, and concentrated under vacuum. The crude (6.0 g) was purified by column chromatography (EA: Hexane) to give 2.5g (~50%) of the desired phosphine oxide (94% desired by HPLC purity). This material was re-purified by column chromatography (EA: Hexane) to afford 1.7 g of pure material (1.7g, HPLC=96.04%). Identification and purity were verified by 1H NMR and HPLC. The remaining fractions were collected and added into the next batch.
References:
US2008/300427,2008,A1 Location in patent:Page/Page column 4
![Phosphine, [(2Z)-2-[(3S,5R)-3,5-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]-2-methylenecyclohexylidene]ethyl]diphenyl-](/CAS/20210305/GIF/127492-07-3.gif)
127492-07-3
0 suppliers
inquiry
![PHOSPHINE OXIDE, [2-[3,5-BIS [[(1,1-DIMETHYLETHYL) DIMETHYLSILY]OXY]-2-METHYLENECYCLOHEXYLIDENE]ETHYL]DIPHENYL-,[3S-(1Z,3A,5B0)]](/CAS/GIF/81522-68-1.gif)
81522-68-1
84 suppliers
inquiry
81506-24-3
31 suppliers
inquiry
![PHOSPHINE OXIDE, [2-[3,5-BIS [[(1,1-DIMETHYLETHYL) DIMETHYLSILY]OXY]-2-METHYLENECYCLOHEXYLIDENE]ETHYL]DIPHENYL-,[3S-(1Z,3A,5B0)]](/CAS/GIF/81522-68-1.gif)
81522-68-1
84 suppliers
inquiry
![Acetic acid, 2-[(3S,5R)-3,5-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]-2-methylenecyclohexylidene]-, ethyl ester, (2E)-](/CAS/20210305/GIF/81506-23-2.gif)
81506-23-2
0 suppliers
inquiry
![PHOSPHINE OXIDE, [2-[3,5-BIS [[(1,1-DIMETHYLETHYL) DIMETHYLSILY]OXY]-2-METHYLENECYCLOHEXYLIDENE]ETHYL]DIPHENYL-,[3S-(1Z,3A,5B0)]](/CAS/GIF/81522-68-1.gif)
81522-68-1
84 suppliers
inquiry
39903-97-4
6 suppliers
inquiry
![PHOSPHINE OXIDE, [2-[3,5-BIS [[(1,1-DIMETHYLETHYL) DIMETHYLSILY]OXY]-2-METHYLENECYCLOHEXYLIDENE]ETHYL]DIPHENYL-,[3S-(1Z,3A,5B0)]](/CAS/GIF/81522-68-1.gif)
81522-68-1
84 suppliers
inquiry
![Cyclohexane, 1-(2-chloroethylidene)-3,5-bis[[(1,1-dimethylethyl)dimethylsilyl]oxy]-2-methylene-, (1Z,3S,5R)-](/CAS/20210111/GIF/81506-25-4.gif)