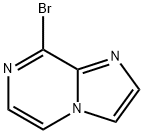
8-Bromoimidazo[1,2-a]pyrazine synthesis
- Product Name:8-Bromoimidazo[1,2-a]pyrazine
- CAS Number:69214-34-2
- Molecular formula:C6H4BrN3
- Molecular Weight:198.02
6863-73-6
216 suppliers
$10.00/5g

17157-48-1
31 suppliers
inquiry
![8-Chloro-imidazo[1,2-a]pyrazine](/CAS/GIF/69214-33-1.gif)
69214-33-1
146 suppliers
$18.00/250mg
![8-Bromoimidazo[1,2-a]pyrazine](/CAS/GIF/69214-34-2.gif)
69214-34-2
44 suppliers
$35.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen bromide in 1,2-dimethoxyethane for 1 h;Heating / reflux;
Steps:
101
α-Bromo diethyl acetal (51.6 mL, 332.7 mmol, 2.5 eq) was added to a solution of 7.7 mL HBr (conc.) and 80 mL of H2O. The reaction was heated at reflux for 1 h. The reaction was cooled and extracted 2× with Et2O (200 mL). The Et2O extracts were combined, washed with brine, and dried over Na2SO4 before being concentrated. The material was not left on the rotavap for an extended time or put under high vacuum. The oily residue was mixed with DME (200 mL) and the 2-amino-3-chloropyrazine (2, 17.240 g, 133.1 mmol) was added. HBr conc. (1-1.5 mL) was added and the reaction was heated at reflux. The reaction is heterogeneous reaction mixture, becomes homogenous after 10-15 minutes. After approximately 30 minutes a precipitate begins to form. After 1 hour at reflux the black reaction was cooled to room temperature, filtered, and washed with Et2O (4×, 75 mL) to give compound 101 1H NMR (DMSO-d6, 400 MHz) 8.70 (d, J=2.0 Hz, 1H), 8.32 (s, 1H), 7.93 (s, 1H), 7.79 (d, J=3.0 Hz, 1H). LC/MS shows a mixture of two products (one product by LC and two by MS). By MS there is a mass for XCl (major) MH+=154 (m/z) and one for XBr (minor) MH+ 198 (m/z). This mixture gave the product in approximately 90% yield as the HBr salt.
References:
Schering Corporation US2007/105864, 2007, A1 Location in patent:Page/Page column 121