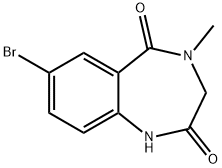
7-BROMO-4-METHYL-3,4-DIHYDRO-1H-BENZO[E][1,4]DIAZEPINE-2,5-DIONE synthesis
- Product Name:7-BROMO-4-METHYL-3,4-DIHYDRO-1H-BENZO[E][1,4]DIAZEPINE-2,5-DIONE
- CAS Number:78756-36-2
- Molecular formula:C10H9BrN2O2
- Molecular Weight:269.09
![4-METHYL-3,4-DIHYDRO-1H-BENZO[E][1,4]DIAZEPINE-2,5-DIONE](/CAS/GIF/3415-35-8.gif)
3415-35-8
28 suppliers
$35.00/200μmol
![7-BROMO-4-METHYL-3,4-DIHYDRO-1H-BENZO[E][1,4]DIAZEPINE-2,5-DIONE](/CAS/GIF/78756-36-2.gif)
78756-36-2
29 suppliers
inquiry
Yield:78756-36-2 75%
Reaction Conditions:
with bromine;sodium acetate;acetic acid at 20; for 24 h;
Steps:
2 Step 2:
Step 2:
Preparation of 7-Bromo-4-methyl-1H-1,4-benzodiazepin-2,5-diaone (4)
To a solution of 1.53 g (8.04 mmol) of 4-methyl-1H-1,4-benzodiazepin-2,5-diaone(3) in 20 mL of acetic acid were slowly added 1.65 g (20.1 mmol) of sodium acetate and 1.03 g (20.1 mmol) of bromine (Br2), followed by stirring the reaction mixture at room temperature for 24 hrs.
Then, the reaction mixture was poured to 200 mL of ice water and filtered.
Purification by flash column chromatography (5% methanol/dichloromethane) afforded 1.58 g of 7-bromo-4-methyl-1H-1,4-benzodiazepin-2,5-diaone (4) as a white solid (75%) (1H NMR (400 MHz, CDCl3) δ 3.29 (s, 3H), 3.90 (s, 2H), 6.91 (d, J=8.4 Hz, 1H), 7.57 (dd, J=8.4, 2.4 Hz, 1H), 8.09 (d, J=2.4 Hz, 1H), 8.77 (s, 1H)).
References:
US2014/81018,2014,A1 Location in patent:Paragraph 0032; 0055; 0056
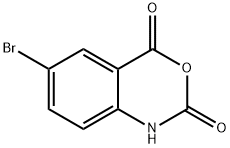
4692-98-2
224 suppliers
$13.00/1g
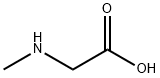
107-97-1
584 suppliers
$5.00/100mg
![7-BROMO-4-METHYL-3,4-DIHYDRO-1H-BENZO[E][1,4]DIAZEPINE-2,5-DIONE](/CAS/GIF/78756-36-2.gif)
78756-36-2
29 suppliers
inquiry

107-97-1
584 suppliers
$5.00/100mg
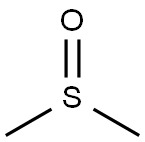
67-68-5
1247 suppliers
$15.00/100g
![7-BROMO-4-METHYL-3,4-DIHYDRO-1H-BENZO[E][1,4]DIAZEPINE-2,5-DIONE](/CAS/GIF/78756-36-2.gif)
78756-36-2
29 suppliers
inquiry
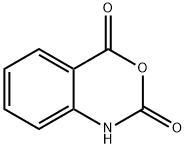
118-48-9
429 suppliers
$5.00/5G
![7-BROMO-4-METHYL-3,4-DIHYDRO-1H-BENZO[E][1,4]DIAZEPINE-2,5-DIONE](/CAS/GIF/78756-36-2.gif)
78756-36-2
29 suppliers
inquiry