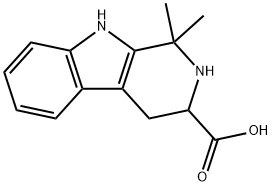
1,1-DIMETHYL-2,3,4,9-TETRAHYDRO-1H-BETA-CARBOLINE-3-CARBOXYLIC ACID synthesis
- Product Name:1,1-DIMETHYL-2,3,4,9-TETRAHYDRO-1H-BETA-CARBOLINE-3-CARBOXYLIC ACID
- CAS Number:73198-03-5
- Molecular formula:C14H16N2O2
- Molecular Weight:244.29
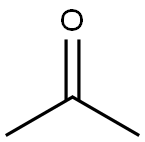
67-64-1
6 suppliers
$21.60/10ml
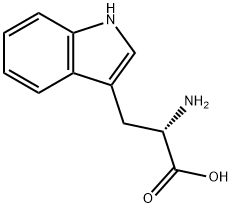
54-12-6
364 suppliers
$6.00/5g

73198-03-5
17 suppliers
$188.00/500mg
Yield:-
Reaction Conditions:
with acetic acid at 80;Pictet-Spengler Synthesis;
Steps:
3.3. Synthesis of derivatives of pyridoindole
General procedure: To develop novel derivatives of pyridoindole scaffold synthesis scheme was designed(Figure 2). Tryptophan as a starting material having an indole ring was used.Bischler-Napieralski or Pictet-Spengler reactions method with some modifications wasdesigned with step I and II (Milen et al. 2005). In step I, DL-Tryptophan was reactedwith required aldehyde or ketone in glacial acetic acid with stirring and heated tillreflux. Different aldehydes and ketones were used and it includes acetaldehyde, benzaldehyde,p-nitrobenzaldehyde, p-cholorobenzaldehyde, p-methoxybenzaldehyde, 2-hydroxybenzaldehyde (salicylaldehyde), ethyl methyl ketone, acetone, diethyl ketone,isopropyl methyl ketone (3-methyl-2-butanone) and propionaldehyde. The reactionmixture was then cooled, was filtered by suction filtration. Residual acetic acid wasremoved by using water and then the product of the first step was dried to give 11products in step 1. At step 2, ethanol and benzyl alcohols were reacted with step 1products in the ice salt bath with the reaction mixture containing SOCl2 for 20 minwith stirring. After that the ice bath was taken aside. The mixture was then slowlyheated to reflux for 4.0 h. The mixture was cooled and stirred into 500 mL of ice water.NaOH solution was used to adjust the pH to neutral and the white precipitate wasformed. Water was used to wash the precipitate, then filtered, and dried using thevacuum to obtain respective novel ethyl/benzyl ester. To check the reaction development,the TLC technique was used. The proposed structures of new synthesised analogueswere confirmed by taking their melting point, IR and NMR spectral information.
References:
Mali, Dipak P.;Gaikwad, Dinanath T.;Bhatia, Manish S.;Bhatia, Neela M. [Natural Product Research,2022,vol. 36,# 11,p. 2767 - 2776]