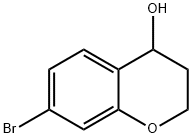
7-BROMOCHROMAN-4-OL synthesis
- Product Name:7-BROMOCHROMAN-4-OL
- CAS Number:18385-82-5
- Molecular formula:C9H9BrO2
- Molecular Weight:229.07
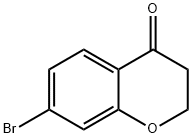
18442-22-3
140 suppliers
$12.00/250mg

18385-82-5
29 suppliers
$116.00/1g
Yield: 98%
Reaction Conditions:
with sodium tetrahydroborate in tetrahydrofuran;methanol at 20; for 0.5 h;
Steps:
96 Synthesis of 7-bromochroman-4-ol
[0331j To a solution of 7-bromochroman-4-one (1.63 g, 7.2 mmol, 1.0 equiv) in MeOH (10 mL) was added NaBH4 (545 mg, 14.4 mmol, 2.0 equiv) and then stirred at room temperature for 30 minutes. After evaporation of the solvent, the residue was purified by silica gel column (EtOAc/hexane=1 :2) to give 7-bromochroman-4-ol (1.61 g, yield: 98%) as a white solid. ESIMS (M+H-1 8) : 211.0. ‘H NMR (400 MHz, CDC13) 5: 7.17 (d, J = 8.0 Hz, 1H), 7.05-7.0 1 (m, 2H), 4.76-4.73 (m, 1H), 4.27-4.24 (m, 2H) 2.14-1.99 (m, 2H).
References:
BIOGEN IDEC MA INC.;SUNESIS PHARMACEUTICALS, INC.;HOPKINS, Brian, T.;MA, Bin;CHAN, Timothy, Raymond;SUN, Lihong;ZHANG, Lei;KUMARAVEL, Gnanasambandam;LYSSIKATOS, Joseph, P.;KOCH, Kevin;MIAO, Hua WO2015/89337, 2015, A1 Location in patent:Paragraph 0331