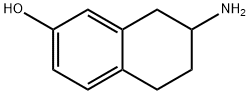
7-AMINO-5,6,7,8-TETRAHYDRO-NAPHTHALEN-2-OL synthesis
- Product Name:7-AMINO-5,6,7,8-TETRAHYDRO-NAPHTHALEN-2-OL
- CAS Number:41363-00-2
- Molecular formula:C10H13NO
- Molecular Weight:163.22
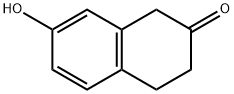
37827-68-2
94 suppliers
$40.00/100mg

41363-00-2
27 suppliers
inquiry
Yield:-
Reaction Conditions:
with methanol;sodium tetrahydroborate;ammonium acetate in methanol at 25;
Steps:
92.1 Step 1 : 3-Aminotetralin-6-ol
To a solution of 7-hydroxytetralin-2-one (500 mg, 3.08 mmol, 1 eq) in anhydrous MeOH (5 mL) was added NH4OAc (7.13 g, 92.49 mmol, 30 eq). After stirring for 5 h, NaBH4 (349.88 mg, 9.25 mmol, 3 eq) was added. The mixture was stirred at 25 °C for 3 h. The mixture was concentrated to remove the solvent. H2O (10 mL) was added, the mixture was extracted with EtOAc (20 mL x 2). The aqueous was adjusted by 3 N HC1 to pH = 6-7, extracted with EtOAc (10 mL x 2). The aqueous was adjusted by 1 N NaOH to pH = 6-7. Then the mixture was lyophilized to give the solid. The solid was dissolved in z'-PrOH (10 mL). The resulting mixture was filtered and concentrated to give 3-aminotetralin-6-ol (550 mg, 2.66 mmol, 86.4% yield, 79.0% purity) as a yellow oil which was used in the next step without further purification. MR (400 MHz, CD3OD) δ ppm 6.91 (d, J = 8.2 Hz, 1H), 6.58 (dd, J = 2.4, 8.4 Hz, 1H), 6.51 (s, 1H), 3.53-3.42 (m, 1H), 3.06 (dd, J = 5.4, 15.5 Hz, 1H), 2.89-2.73 (m, 3H), 2.17 (d, J = 10.1Hz, 1H), 1.84-1.74 (m, 1H); ES-LCMS m/z 164.3 [M+H].
References:
WO2018/195397,2018,A2 Location in patent:Paragraph 00644-00645
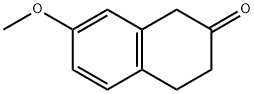
4133-34-0
280 suppliers
$15.00/1g

41363-00-2
27 suppliers
inquiry
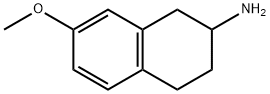
4003-89-8
86 suppliers
inquiry

41363-00-2
27 suppliers
inquiry
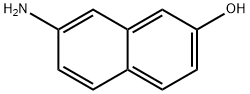
93-36-7
27 suppliers
inquiry

41363-00-2
27 suppliers
inquiry
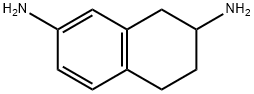
103393-75-5
11 suppliers
inquiry

41363-00-2
27 suppliers
inquiry