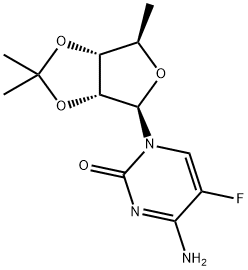
5'-Deoxy-5-fluoro-2',3'-O-isopropylidene-D-cytidine synthesis
- Product Name:5'-Deoxy-5-fluoro-2',3'-O-isopropylidene-D-cytidine
- CAS Number:66335-37-3
- Molecular formula:C12H16FN3O4
- Molecular Weight:285.27
Yield:-
Reaction Conditions:
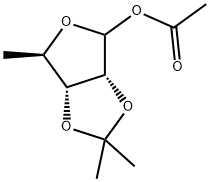
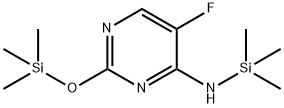
Stage #1: 2,3-O-isopropylidene-3-O-acetyl-5-deoxy-D-ribose;2-trimethylsilyloxy-4-trimethylsilylamino-5-fluoropyrimidine in dichloromethane at 0 - 5; for 0.166667 h;
Stage #2: with tin(IV) chloride in dichloromethane at 0 - 30; for 2 h;
Steps:
6
The obtained reaction residue (silylated compound) was cooled to 0-5 °C. Dissolved 1 g of 2,3-0- isopropylidene-3-O-acetyl-5-deoxy-D-ribose of Formula III in dichloromethane (2 ml) and then added dropwise to the above silylated residue at 0-5 0C over 10 minutes. Stannic chloride (0.6 ml) was charged to the above reaction suspension at 0-5 0C. The reaction solution obtained was allowed to reach a temperature of 25-30 0C followed by stirring for 2 hours. Conversion of the reactants to product was monitored by TLC. After completion of the reaction, sodium bicarbonate (1.6 g) was charged to above reaction solution and then demineralized water (0.6 ml) was added. The reaction suspension was stirred at 25-30 0C for 2 hours and the suspension was filtered through a celite bed and the filtrate was washed with 5 % aqueous sodium bicarbonate solution (10 ml). The organic solution was dried over anhydrous sodium sulfate and then distilled completely at 45 0C under a vacuum of 600 mm Hg. The residue obtained was purified by column chromatography using 10 % methanol in dichloromethane as eluent to afford 0.4 g of the title compound. Mass: m/z 286.2[m++H]H'NMR: δ 1.33 (d, 3H), 1.45 (s, 6H), 4.01 (q, 1 H), 4.7(d, 1 H)1 4.9 (d, 1 H), 5.7 (s, 1 H), 7.5-7.8 (2H), 7.9 (d, 1 H)
References:
WO2008/131062,2008,A2 Location in patent:Page/Page column 35