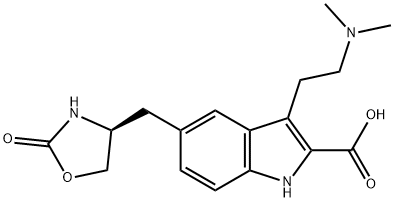
(S)-3-(2-(dimethylamino)ethyl)-5-((2-oxooxazolidin-4-yl)methyl)-1H-indole-2-carboxylic acid synthesis
- Product Name:(S)-3-(2-(dimethylamino)ethyl)-5-((2-oxooxazolidin-4-yl)methyl)-1H-indole-2-carboxylic acid
- CAS Number:659738-69-9
- Molecular formula:C17H21N3O4
- Molecular Weight:331.37
![Zolmitriptan Related Compound D (20 mg) ((S)-Ethyl 3-[2-(dimethylamino)ethyl]-5-[(2-oxooxazolidin-4-yl)methyl]-1H-indole-2-carboxylate)](/CAS/20150408/GIF/191864-24-1.gif)
191864-24-1
39 suppliers
inquiry

659738-69-9
20 suppliers
inquiry
Yield:659738-69-9 94%
Reaction Conditions:
with potassium hydroxide;water in ethanol; for 1 h;Heating / reflux;
Steps:
7 Example 7: [(S)-3- (2-DIMETHYLAMINOETHYL)-5- (2-OXO-] 1, [3-OXAZOLIDIN-4-YLMETHYL)-LH-INDOL-2-CARBOXYLIC] acid
To a solution of [1.] 4 g (24.9 mmoles) of KOH in 30 ml of ethanol was added 2.8 g (7.8 mmoles) of [(S)-3- (2-] [DIMETHYLAMINOETHYL)-5-[2-OXO-1, 3-OXAZOLIDIN-4-YLMETHYL]-] [LH-INDOL-2-CARBOXYLIC] acid ethyl ester. The resulting solution was heated at reflux temperature for one hour. It was cooled and the solvent evaporated to dryness. The residue was dissolved in 6 ml of water and washed three times with 10 ml of dichloromethane. The aqueous solution was cooled to [5°C,] adjusted to pH 6 with glacial acetic acid, stirred for 30 minutes at that temperature and the water evaporated to dryness. The residue'was redissolved in 30 ml of water and 5 g of ionic exchange resin (Dowex [50WX8-400)] added. The mixture was left under stirring at room temperature for 24 hours. The was filtered and it was washed with water. For desorption the resin was suspended with 20 ml of a 10% aqueous solution of ammonia and stirred at room temperature for 5 hours. Once that time had elapsed it was filtered and washed with water. The water was evaporated to dryness under reduced pressure to give 7.75 g [(94%)] of the title acid as a yellow crystalline solid. M. p. [230 °C.] IR (KBr): 1342 [CM-1,] 1403 [CM 1,] 1587 [CM'] 1739 [CM~1] 3430 cm [H-NMR] (200 MHz, DMSO-d6) : 2.47 (s, 6H. N (CH3) [2),] 2.87 (m, 4H, [CH2C_2N),] 3.27 (m, 2H, CH2-benz.) ; 4.03 (m, 2H, [OCH2)] ; 4.27 (m, 1H, NHCH), 6.98 (d, [J=8,] 0 Hz, 1H, ar), 7.27 (d, [J=8,] 0 Hz, 1H, ar), 7.41 (s, 1H, ar) ; 7.83 (s, 1H, CONH), 10.95 (s, 1H, [NH-INDOLE).] [3C-NMR] (200 MHz, DMSO-d6) : 21.15 ; 43. 7 ; 53.3 ; 59. [7 ;] 68.2 ; 112.1 ; 113.2 ; 119. 7 ; 124. 4 ; 126.4 ; 128. 1 ; 132.2 ; 134.0 ; 158.9 ; 166. [2.]
References:
WO2004/14901,2004,A1 Location in patent:Page 17-18