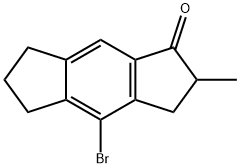
4-bromo-2-methyl-2,3,6,7-tetrahydros-indacen-1(5H)-one synthesis
- Product Name:4-bromo-2-methyl-2,3,6,7-tetrahydros-indacen-1(5H)-one
- CAS Number:656800-67-8
- Molecular formula:C13H13BrO
- Molecular Weight:265.15
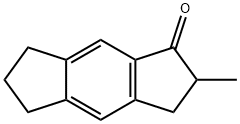
202667-44-5
35 suppliers
inquiry

656800-67-8
17 suppliers
inquiry
Yield:656800-67-8 54%
Reaction Conditions:
with bromine;aluminum (III) chloride at 76; for 2.5 h;
Steps:
1.1b
3,5,6,7-tetrahydro-2-methyl-s-hydraindacen-1(2H)-one (50.24 g, 0.270 moles) was gradually added over one hour with stirring to an oven dried, nitrogen purged, 1L glass round bottom flask containing AlCl3 (99. 842 g, 0.749 mol). Bromine (13.8 mL, 0.268 mol) was added via a dropping funnel over 45 minutes. The resulting red mixture was heated with stirring to 76°C for 45 minutes. The reaction mixture was cooled to room temperature and poured onto ice (1500 g) containing concentrated hydrochloric acid (50 mL) and then extracted with diethylether (4 x 200 mL). The organic fractions were combined, washed with aqueous NaHCO3 and water, dried over MgSO4, and dried under dynamic vacuum. The mixture was then fractionally distilled. The fraction obtained at 135-142°C @ 15 mTorr was found to be the desired product in greater than 90 percent purity. Yield: 20.4 g, 54 percent.
References:
WO2004/13149,2004,A1 Location in patent:Page/Page column 12-13
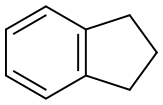
496-11-7
243 suppliers
$5.00/5g

656800-67-8
17 suppliers
inquiry