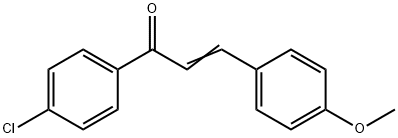
1-(4-CHLOROPHENYL)-3-(4-METHOXYPHENYL)PROP-2-EN-1-ONE synthesis
- Product Name:1-(4-CHLOROPHENYL)-3-(4-METHOXYPHENYL)PROP-2-EN-1-ONE
- CAS Number:6552-63-2
- Molecular formula:C16H13ClO2
- Molecular Weight:272.73
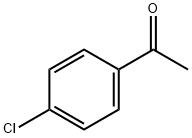
99-91-2
435 suppliers
$10.00/25g
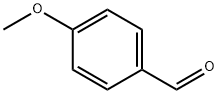
123-11-5
866 suppliers
$10.00/10g

6552-63-2
45 suppliers
$21.00/250mg
Yield:6552-63-2 96.9%
Reaction Conditions:
with methanol;sodium in methanol at 20;Aldol Condensation;
Steps:
General Procedure for the Synthesis of α,β-unsaturated Carbonyl Compounds (2a-2o).
General procedure: Na (0.01 g, 0.4 mmol) was cut to pieces and added into anhydrous MeOH (15 mL) under ice bath; the fresh MeONa/MeOH solution was prepared till no gas evolved. 4-Methoxybenzaldehyde (1.36 g, 10.0 mmol) was added to the fresh, cooled MeONa/MeOH solution (15 mL), and then 10.0 mmol acetyl aromatic compound was added dropwise. The mixture was stirred at room temperaturefor2-8 h(36-54 hfor2nand2oduetothesteric hindrance of thenaphthyl andbiphenyl). Theend ofthereaction was monitored by TLC. Afterward, water (20 mL) and EtOAc (75 mL, 25 mL × 3) were added into the mixture. Theinorganic precipitatewas removed, and theorganic solution was washed with brine three times and dried. The crude product was puried by silica gel column chromatography with EtOAc/hexane as eluent to get pureα,β-unsaturated carbonyl compounds (2a-2o).
References:
Zhan, Xiaoping;Lan, Lan;Zhang, Yuankui;Chen, Jian;Zhao, Kai;Wang, Shuai;Xin, Yuxuan;Mao, Zhenmin [Bulletin of the Korean Chemical Society,2016,vol. 37,# 2,p. 200 - 206]
90956-78-8
0 suppliers
inquiry

6552-63-2
45 suppliers
$21.00/250mg
104-46-1
334 suppliers
$5.00/100mg

6552-63-2
45 suppliers
$21.00/250mg
104-88-1
625 suppliers
$5.00/25g
100-06-1
593 suppliers
$22.10/25g

6552-63-2
45 suppliers
$21.00/250mg
![Benzene, 1-chloro-4-[1-[(trimethylsilyl)oxy]ethenyl]-](/CAS/20200401/GIF/58518-76-6.gif)
58518-76-6
0 suppliers
inquiry

123-11-5
866 suppliers
$10.00/10g

6552-63-2
45 suppliers
$21.00/250mg