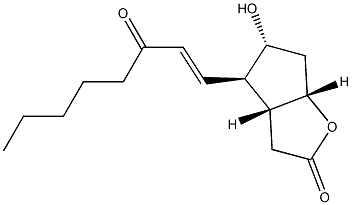
(3aR,4R,5R,6aS)-Hexahydro-5-hydroxy-4-[(1E)-3-oxo-1-octen-1-yl]-2H-cyclopenta[b]furan-2-one synthesis
- Product Name:(3aR,4R,5R,6aS)-Hexahydro-5-hydroxy-4-[(1E)-3-oxo-1-octen-1-yl]-2H-cyclopenta[b]furan-2-one
- CAS Number:60623-67-8
- Molecular formula:C15H22O4
- Molecular Weight:266.3328
Yield:60623-67-8 87%
Reaction Conditions:
Stage #1: dimethyl 2-oxoheptylphosphonatewith potassium hexamethylsilazane in tetrahydrofuran at -78; for 0.5 h;Inert atmosphere;
Stage #2: Corey aldehyde in tetrahydrofuran at 20; for 4 h;Temperature;Reagent/catalyst;
Steps:
1.2; 2.2; 3.2; 4.2; 5.2; 6.2; 7.2; 8.2; 9.2 (2) Synthesis of compound 3
Suspend dimethyl (β-oxoheptyl)phosphonate (10g, 45mmol) in anhydrous THF (50mL) under nitrogen protection at -78°C, and mix KHMDS (45mL, 45mmol, 1M) in THF (100mL) solution Add dropwise to the above suspension. After stirring at -78°C for 30 min, a solution of compound 2 (3.8 g, 22 mmol) in THF (20 mL) was slowly added dropwise to the above suspension. Then, the reaction temperature was slowly raised to room temperature, and the reaction was stirred for 4 hours. After the reaction is complete, add saturated NH4Cl solution (50mL) to quench the reaction, extract with ethyl acetate (200mL×3), combine the organic phases, wash with saturated brine, dry with anhydrous Na2SO4, filter, concentrate, and separate and purify by column chromatography to obtain Light yellow oil (6.9 g, 87%).
References:
CN111777537,2020,A Location in patent:Paragraph 0090; 0094-0096; 0118; 0122-0124; 0146; 0150-0152
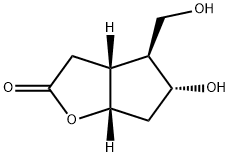
32233-40-2
364 suppliers
$9.00/250mg
![(3aR,4R,5R,6aS)-Hexahydro-5-hydroxy-4-[(1E)-3-oxo-1-octen-1-yl]-2H-cyclopenta[b]furan-2-one](/CAS/20150408/GIF/60623-67-8.gif)
60623-67-8
2 suppliers
inquiry
![2H-Cyclopenta[b]furan-2-one, hexahydro-4-[(1E)-3-oxo-1-octen-1-yl]-5-[(triethylsilyl)oxy]-, (3aR,4R,5R,6aS)-](/CAS/20210111/GIF/128948-11-8.gif)
128948-11-8
2 suppliers
inquiry
![(3aR,4R,5R,6aS)-Hexahydro-5-hydroxy-4-[(1E)-3-oxo-1-octen-1-yl]-2H-cyclopenta[b]furan-2-one](/CAS/20150408/GIF/60623-67-8.gif)
60623-67-8
2 suppliers
inquiry
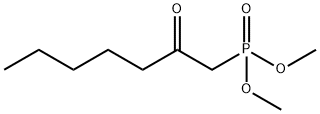
36969-89-8
146 suppliers
$16.00/250mg
![(3aR,4R,5R,6aS)-Hexahydro-5-hydroxy-4-[(1E)-3-oxo-1-octen-1-yl]-2H-cyclopenta[b]furan-2-one](/CAS/20150408/GIF/60623-67-8.gif)
60623-67-8
2 suppliers
inquiry
![2H-Cyclopenta[b]furan-4-carboxaldehyde, hexahydro-2-oxo-5-[(triethylsilyl)oxy]-, (3aR,4R,5R,6aS)-](/CAS/20200611/GIF/128948-10-7.gif)
128948-10-7
0 suppliers
inquiry
![(3aR,4R,5R,6aS)-Hexahydro-5-hydroxy-4-[(1E)-3-oxo-1-octen-1-yl]-2H-cyclopenta[b]furan-2-one](/CAS/20150408/GIF/60623-67-8.gif)
60623-67-8
2 suppliers
inquiry
![2H-Cyclopenta[b]furan-4-carboxaldehyde, hexahydro-5-hydroxy-2-oxo-, (3aR,4R,5R,6aS)-](/CAS/20200611/GIF/62961-72-2.gif)