
6-NITRO INDAZOLE-3-CARBOXALDEHYDE synthesis
- Product Name:6-NITRO INDAZOLE-3-CARBOXALDEHYDE
- CAS Number:315203-37-3
- Molecular formula:C8H5N3O3
- Molecular Weight:191.14
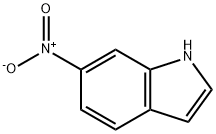
4769-96-4
266 suppliers
$9.00/250mg

315203-37-3
88 suppliers
$45.00/1mg
Yield:315203-37-3 77%
Reaction Conditions:
Stage #1: 6-nitroindolewith sodium nitrite in water at 20;
Stage #2: with hydrogenchloride in water at 20; for 1.5 h;
Steps:
4.4.4. 6-Nitro-1H-indazole-3-carbaldehyde (6)
To a 500 mL round bottomed flask with stir bar was addedNaNO2 (6.38 g, 92.5 mmol) and H2O (150 mL). 6-Nitroindole (5,1.5 g, 9.3 mmol) was added to the above solution at 20 C andthe resultant mixture was stirred vigorously until evenly suspended(over 5 min.). To this bright yellow suspension was added6 M HCl (14 mL) via addition funnel over 30 min. at 20 C, and theresultant suspension was stirred for 90 min. A sample aliquot wastaken from the reaction, filtered, and the precipitate was dissolvedin a minimal amount of HPLC grade MeCN. Analysis with LC-MSconfirmed reaction completion. The product was vacuum filteredand the precipitate was washed with additional H2O (50 mL). Theprecipitate was dried to give 6 (1.37 g) as an orange solid in 77%yield. mp: changed color from orange to brown between 120 and160 C, decomposed after 200 C; LC/MS tR = 2.40 min (CharacterizationMethod A); m/z = 190.05 (MH+); 1H NMR (400 MHz,DMSO-d6) d = 10.22 (s, 1H), 8.57 (d, J = 1.5 Hz, 1H), 8.29 (dd, J = 0.5,8.9 Hz, 1H), 8.13 (dd, J = 2.0, 8.9 Hz, 1H); 13C NMR (100 MHz,DMSO-d6) d = 187.7, 146.9, 124.0, 122.4, 118.7, 108.6.
References:
Gandhi, Disha M.;Majewski, Mark W.;Rosas, Ricardo;Kentala, Kaitlin;Foster, Trevor J.;Greve, Eric;Dockendorff, Chris [Bioorganic and Medicinal Chemistry,2018,vol. 26,# 9,p. 2514 - 2529]
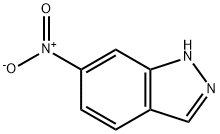
7597-18-4
302 suppliers
$17.00/25g

315203-37-3
88 suppliers
$45.00/1mg