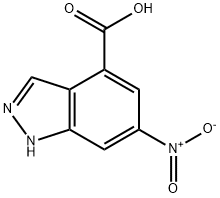
6-Nitro-1H-indazol-4-carboxylic acid synthesis
- Product Name:6-Nitro-1H-indazol-4-carboxylic acid
- CAS Number:885519-61-9
- Molecular formula:C8H5N3O4
- Molecular Weight:207.14
885518-55-8
58 suppliers
$56.00/100mg

885519-61-9
22 suppliers
inquiry
Yield:-
Reaction Conditions:
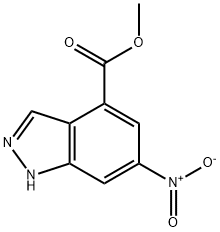
Stage #1: methyl 6-nitro-1H-indazole-4-carboxylatewith water;sodium hydroxide in tetrahydrofuran;methanol at 70; for 1 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;methanol;water;
Steps:
51.1
First Step [Show Image] Methyl 6-nitro-1H-indazole-4-carboxylate (see Example 41) (3.02 g, 13.65 mmol) was dissolved in a THF/methanol 1:1 mixed solution (200 mL) and an aqueous 2N sodium hydroxide solution (35 mL) was added, followed by stirring at 70°C for one hour. The reaction mixture was acidified by adding 2N hydrochloric acid and then extracted with ethyl acetate. The organic layer was washed with a saturated brine solution and dried over anhydrous sodium sulfate, and then solvent was distilled off under reduced pressure to obtain 2.86 g of 6-nitro-1H-indazole-4-carboxylic acid. To an anhydrous toluene solution (250 mL) of this product, triethylamine (1.73 g, 17.06 mmol) was added under a nitrogen gas flow, and then diphenylphosphoric acid azide (4.70 g, 17.06 mmol) was added. After the reaction mixture was refluxed for one hour, ethanol (3.14 g, 68.3 mmol) was added, followed by reflux for 2 hours. After cooling to room temperature, the reaction mixture was diluted with ethyl acetate, washed in turn with an aqueous 1 N sodium hydroxide solution and water and then dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain 3.42 g of ethyl 6-nitro-1H-indazole-4-carbamate. 1H-NMR (400 MHz, DMSO-d6) d: 1.32 (t, 3H, J = 7.2 Hz), 4.25 (q, 2H, J = 7.2 Hz), 8.09 (s, 1H), 8.48 (d, 1H, J = 1.6 Hz), 8.64 (s, 1H), 10.40 (s, 1H), 13.75 (br, 1H).
References:
EP2226315,2010,A1 Location in patent:Page/Page column 54