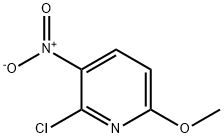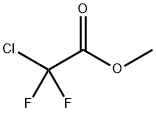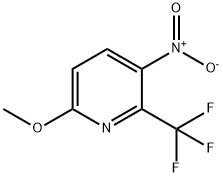
6-Methoxy-2-trifluoromethyl-3-nitropyridine synthesis
- Product Name:6-Methoxy-2-trifluoromethyl-3-nitropyridine
- CAS Number:727993-33-1
- Molecular formula:C7H5F3N2O3
- Molecular Weight:222.12
Yield:727993-33-1 79%
Reaction Conditions:
Stage #1: 2-chloro-6-methoxy-3-nitropyridine;metyhyl chlorodifluoroacetatewith potassium fluoride;copper(l) iodide in N,N-dimethyl-formamide at 125; for 13 h;
Stage #2: with ammonia;ammonium chloride in water;N,N-dimethyl-formamide at 20; for 1.5 h;
Steps:
2.1
Step 1: The reaction was performed in three identical experiments of equal size: 2-chloro-6-methoxy-3-nitropyridine (altogether 15.0 g, 79.6 mmol), copper(I)-iodide (6.06 g, 31.8 mmol, 1.2 eq.) and potassium fluoride (3.08 g, 53.0 mmol, 2.0 eq.) were suspended in DMF (30 ml), and methyl chlorodifluoroacetate (7.1 ml, 66.3 mmol, 2.5 eq.) was added. The mixture was stirred at 125°C for 13 h and was allowed to cool to room temperature. The reaction mixture was poured into a mixture of conc. aq. NH3-solution (140 ml) and saturated aq. NH4Cl-solution (210 ml), and the deep-blue solution was stirred for 1.5 h at room temperature. Extraction with EA (3x150ml) was followed by washing of the combined organic layer with 1/4-saturated NaCl-solution (100 ml) and brine (2*50 ml), drying over Na2SO4 and evaporation of the solvent. The crude product (20 g) was purified by flash chromatography (eluent hexane/DCM 6:1 to 4:1) to give 14.0 g (62.9 mmol, 79%) of 6-methoxy-3-nitro-2-(trifluoromethyl)pyridine as a yellow oil. TLC (hexane/DCM 4:1) Rf: 0.29. 1H-NMR (method B, DMSO-d6): 8.53 (dd, J = 0.5, 9.0, 1H), 7.39 (dd, J = 0.4, 9.0, 1H), 4.02 (s, 3H).
References:
EP2128158,2009,A1 Location in patent:Page/Page column 20-21