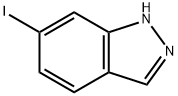
6-Iodo-1H-indazole synthesis
- Product Name:6-Iodo-1H-indazole
- CAS Number:261953-36-0
- Molecular formula:C7H5IN2
- Molecular Weight:244.03
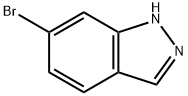
79762-54-2
374 suppliers
$3.00/1g

261953-36-0
259 suppliers
$9.00/250mg
Yield:261953-36-0 85%
Reaction Conditions:
with copper(l) iodide;tetra-(n-butyl)ammonium iodide;N,N-dimethylethylenediamine;potassium iodide in 1,4-dioxane for 48 h;Reflux;Reagent/catalyst;Solvent;
Steps:
1 Example 1 A method of synthesizing 6-iodo -1H- indazole, the following synthetic route:
The method of synthesizing the above-described 6-iodo-1H-indazole, comprising the steps of: Weigh 5.0 g of the compound of formula V and 13.38 g of potassium iodide in the reactor.After adding 50 mL of 1,4-dioxane, 0.2 g of tetrabutylammonium iodide was added, based on the compound of formula V.0.51 g of cuprous iodide and 0.47 g of N,N-dimethylethylenediamine (0.2 equiv.) were added to the reactor, the temperature was raised to reflux, and the reaction was refluxed for 48 h.After the reaction was completed, after cooling to room temperature, the reaction solution was filtered, and the filter cake was washed three times with 1,4-dioxane.The filtrate was combined, and the obtained filtrate was concentrated. After the concentrate was dissolved in ethyl acetate, ethyl acetate layer was washed with 13% aqueous ammonia.The organic phase is separated, the organic phase is concentrated, and the concentrate is recrystallized from acetonitrile.To give compound of formula 6-iodo-1H-indazole in 85% yield,
References:
Anhui Nuoquan Pharmaceutical Co., Ltd.;Qian Zhujin;Hu Zhigang;Xu Liangzhi;He Darong;Du Xiaopeng;He Yong;Chen Yuelei CN109761904, 2019, A Location in patent:Paragraph 0027-0046; 0050; 0051
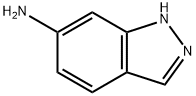
6967-12-0
198 suppliers
$9.00/1g

261953-36-0
259 suppliers
$9.00/250mg
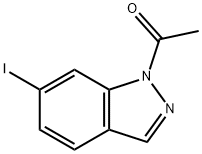
850363-52-9
8 suppliers
inquiry

261953-36-0
259 suppliers
$9.00/250mg
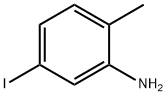
83863-33-6
217 suppliers
$5.00/250mg

261953-36-0
259 suppliers
$9.00/250mg
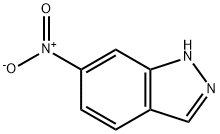
7597-18-4
302 suppliers
$17.00/25g

261953-36-0
259 suppliers
$9.00/250mg