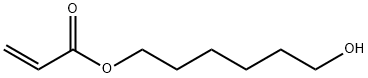
6-hydroxyhexyl acrylate synthesis
- Product Name:6-hydroxyhexyl acrylate
- CAS Number:10095-14-4
- Molecular formula:C9H16O3
- Molecular Weight:172.22
Yield: 242 g
Reaction Conditions:
with 10H-phenothiazine;dioctyltin(IV) oxide in cyclohexane at 85;Concentration;Temperature;
Steps:
V-5
To a 2 L four-necked glass flask were attached a 20-stage Oldershaw, a reflux condenser and a gas introductiontube, and the flask was charged with 886.6 g (7.5 moles) of 1,6-hexanediol, then 430.45 g (5.0 moles) of methyl acrylate,100 g of cyclohexane and 0.721 g of phenothiazine as a polymerization inhibitor. Thereafter, 3.61 g of dioctyltin oxideas a catalyst for transesterification was added to the flask. While a nitrogen gas containing 7% by volume of oxygenwas blown into the flask at a flow rate of 20 mL/min, the reaction of 1,6-hexanediol and methyl acrylate was carried outat 85°C. In addition, the azeotropic mixture of methanol and cyclohexane by-products generated in the transesterificationof 1,6-hexanediol and methyl acrylate was taken out from the distillation tower as required, and introduced into a heatable500 mL decanter. To the decanter, 50 mL of 1,6-hexanediol heated to 45°C was gradually added, and the alcohol solutioncontaining separated methanol and 1,6-hexanediol was taken out from the decanter, to separate 200 g of the alcoholsolution from cyclohexane. The transesterification reaction of 1,6-hexanediol and methyl acrylate was carried out at 85°C until almost nomethanol was distilled out, so that the transesterification reaction of 1,6-hexanediol and methyl acrylate was terminatedto obtain a reaction mixture. The obtained reaction mixture was heated to 88°C, and unreacted methyl acrylate was removed by azeotropicdistillation with cyclohexane, to obtain 1285 g of crude 6-hydroxyhexyl acrylate. Next, the obtained crude 6-hydroxyhexyl acrylate was collected by extraction, and further subjected to thin filmdistillation, to obtain 242 g of refined 6-hydroxyhexyl acrylate. On the other hand, 200 g of the alcohol solution taken out from the decanter was heated to 80°C, and thedegree of pressure reduction was gradually increased, and finally the degree of pressure reduction was adjusted to 250kPa, to remove methanol by distillation. As a result, the amount of methanol removed by distillation was 115.9 g, andthe amount of remaining 1,6-hexanediol was 83.3 g. Accordingly, it can be understood that methanol which is generatedas a by-product in the transesterification of 1,6-hexanediol and methyl acrylate can be efficiently collected. Additionally, the collected methanol was analyzed by gas chromatography, As a result, a notable peak attributedto methanol was observed, and slight peaks attributed to cyclohexane and methyl acrylate were also observed. Accordingly,it was confirmed that methanol which is a by-product having a relatively high purity can be easily collected, andthat the methanol can be effectively used not only as a raw material for organic compound synthesis but also as a fuel. From the above-mentioned results, it can be understood that according to the process for preparing a hydroxyalkylacrylate of the present invention, not only it is made possible to efficiently collect the alcohol generated as a byproductat the time of preparing a hydroxyalkyl acrylate by a transesterification method, but also it is made possible toreuse a polyhydric alcohol separated from the by-product alcohol in the transesterification reaction of a polyhydric alcohol and an acrylic acid alkyl ester, to give a hydroxyalkyl acrylate.
References:
Osaka Organic Chemical Ind., Ltd.;SUGIURA, Toyoyuki;OKAMURA, Kohei;ITO, Keisuke;KAWAKAMI, Naohiko;MATSUMOTO, Shigeaki;MATSUNO, Masayoshi EP2860170, 2015, A1 Location in patent:Paragraph 0524; 0525; 0526; 0527; 0528; 0529; 0530; 0531
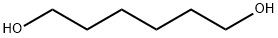
629-11-8
449 suppliers
$6.00/25g
292638-85-8
3 suppliers
inquiry
13048-33-4
322 suppliers
$7.00/25g

10095-14-4
15 suppliers
inquiry
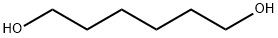
629-11-8
449 suppliers
$6.00/25g
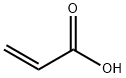
79-10-7
698 suppliers
$20.16/100 mL

10095-14-4
15 suppliers
inquiry