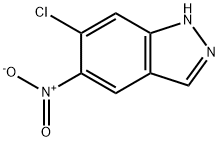
6-CHLORO-5-NITRO-1H-INDAZOLE synthesis
- Product Name:6-CHLORO-5-NITRO-1H-INDAZOLE
- CAS Number:101420-98-8
- Molecular formula:C7H4ClN3O2
- Molecular Weight:197.58
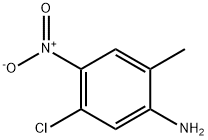
13852-51-2
151 suppliers
$8.00/250mg

101420-98-8
85 suppliers
inquiry
Yield:101420-98-8 80%
Reaction Conditions:
Stage #1: 1-methyl-2-amino-4-chloro-5-nitro-benzenewith hydrogenchloride;NaNO2 in lithium hydroxide monohydrate at 0; for 0.333333 h;
Stage #2: with sodium tetrafluoroborate in lithium hydroxide monohydrate; for 0.666667 h;Cooling;
Stage #3: with anhydrous potassium acetate in chloroform at 20; for 2 h;
Steps:
1.1.1 Step 1: Synthesis of Deuterated Compound 4a
(1)Compound 1 (18.6 g, 0.1 mol) was suspended in water (80 ml) and concentrated hydrochloric acid (25 ml, 0.3 mol). At 0 °C, an aqueous NaNO2 solution (6.9 g, 0.1 mol) was added dropwise to the above solution, Then stirred for 20min, filtered, pre-cooled NaBF4 (12.g, 0.1 mol) aqueous solution (40ml) was added to the above filtrate, stirred for 40min. Stop stirring, filter, wash the filter cake with cold ethanol and diethyl ether, collect the filter cake, and dry to obtain the diazonium salt (11.5 g, 0.05 mol).The diazonium salt was dissolved in CHCl3 (100 mL), KOAc (8.15 g, 0.78 mol) was added to the above solution, stirred at room temperature for 2 h, the reaction was complete, and the stirring was stopped.Water (50 ml) was added to quench the reaction, extracted with DCM (50 mL × 3), the organic phases were combined, washed with saturated brine, dried over anhydrous Na2SO4, filtered, concentrated, and recrystallized to obtain compound 2 (15.68 g, 80%).
References:
CN114507221,2022,A Location in patent:Paragraph 0045
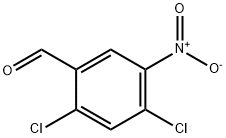
53581-87-6
24 suppliers
$110.00/100mg

101420-98-8
85 suppliers
inquiry
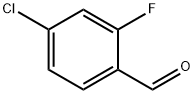
61072-56-8
287 suppliers
$14.00/5g

101420-98-8
85 suppliers
inquiry
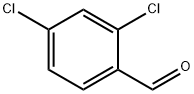
874-42-0
413 suppliers
$6.00/25g

101420-98-8
85 suppliers
inquiry