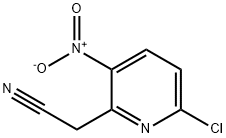
(6-Chloro-3-nitro-pyridin-2-yl)-acetonitrile synthesis
- Product Name:(6-Chloro-3-nitro-pyridin-2-yl)-acetonitrile
- CAS Number:123846-69-5
- Molecular formula:C7H4ClN3O2
- Molecular Weight:197.58
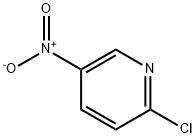
4548-45-2
553 suppliers
$6.00/5g
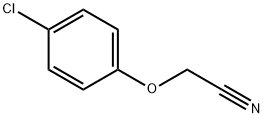
3598-13-8
129 suppliers
$17.00/10g

123846-69-5
32 suppliers
inquiry
Yield:123846-69-5 7%
Reaction Conditions:
with hydrogenchloride in tetrahydrofuran;dichloromethane;acetic acid;
Steps:
3.A A.
A. (5-Amino-2-chloro-4-pyridyl) acetonitrile To a stirred solution of potassium t-butoxide (24.69 g, 220 mmol, 2.2 eq) in anhydrous tetrahydrofuran (150 ml) at -50° C. under nitrogen, a solution of 2-chloro-5-nitropyridine (15.85 g, 100 mmol) and (4-chlorophenoxy)acetonitrile (E. Grochowski et al., Bull. Acad. Pol. Sci. Ser. Sci. Chim., 11, 443 (1963)) (18.44 g, 110 mmol, 1.1 eq) in anhydrous tetrahydrofuran (150 ml) was added dropwise at such a rate that the reaction temperature was maintained at -40° to -50° C. with cooling in a dry ice/acetone bath. The resultant purple colored reaction mixture was then stirred at -78° C. under nitrogen for 1 hour, at which time glacial acetic acid (20 ml, 0.35 mol, 3.5 eq) was added to the reaction, and the mixture was allowed to warm to room temperature. A solution of 5% HCl (100 ml) was added to the reaction mixture and this aqueous mixture was extracted with ethyl ether (100 ml) and then with methylene chloride (2*100 ml). The extracts were combined, dried over magnesium sulfate, and passed through a silica gel filter (approximately 150 g) followed by methylene chloride (1200 ml). This filtrate was evaporated under reduced pressure, and the residual oil was chromatographed using silica gel (approximately 300 g) and eluted with 25% hexanes in methylene chloride to afford an oil (Rf =0.52 in methylene chloride) which was triturated in cold anhydrous ether to afford 6-chloro-3-nitro-2-pyridyl acetonitrile (1.37 g, 7%) as a white crystalline solid: mp, 121.5°-123.5° C.
References:
US5811432,1998,A
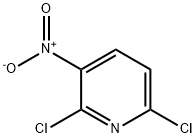
16013-85-7
503 suppliers
$9.00/5g

123846-69-5
32 suppliers
inquiry