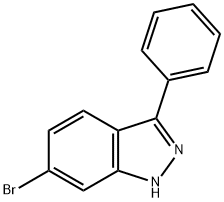
6-BROMO-3-PHENYL-1H-INDAZOLE synthesis
- Product Name:6-BROMO-3-PHENYL-1H-INDAZOLE
- CAS Number:885271-16-9
- Molecular formula:C13H9BrN2
- Molecular Weight:273.13
135776-98-6
76 suppliers
inquiry

885271-16-9
32 suppliers
inquiry
Yield:885271-16-9 42%
Reaction Conditions:
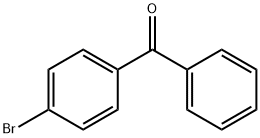
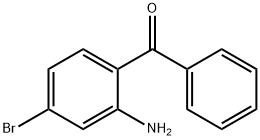
Stage #1: (4-bromo-2-aminophenyl)phenyl-methanonewith hydrogenchloride;sodium nitrite in water at 0; for 0.55 h;
Stage #2: with hydrogenchloride;tin(ll) chloride in water at 0 - 20; for 1.16667 h;
Stage #3: with sodium hydroxide in water;
Steps:
112.a
EXAMPLE 112; 2,2-Dimethyl-3-phenyl-3-(3-phenyl-lH-indazol-6-yl)-N-(thiazol-2- yl)propanamide; (a) To a stirred mixture of (2-amino-4-bromophenyl)(phenyl)methanone (1.0 g, 3.6 mmol) in hydrochloric acid (6N aqueous solution, 5 mL) was added sodium nitrite (0.5 g, 7.2 mmol) over 3 min at 00C. The mixture was stirred at 00C for 30 min before a solution of tin(II) chloride (2.7 g, 14.4 mmol in 5 mL concentrated hydrochloric acid) was added at 00C over 5 min. After stirring at 00C for 10 min and rt for 1 hr, the reaction mixture was filtered. The solid was washed with water (10 mL), 4N aqueous sodium hydroxide solution, and water (10 mL). The solid was dissolved in dichloromethane (20 mL), washed with brine (10 mL), dried (Na2SO4), and concentrated. Silica gel flash chromatography gave 6-bromo-3 -phenyl- IH- indazole (0.42 g, 1.5 mmol, 42% yield).
References:
WO2008/57856,2008,A2 Location in patent:Page/Page column 123