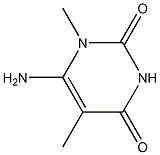
6-amino-1,5-dimethylpyrimidine-2,4(1H,3H)-dione synthesis
- Product Name:6-amino-1,5-dimethylpyrimidine-2,4(1H,3H)-dione
- CAS Number:1609259-60-0
- Molecular formula:C6H9N3O2
- Molecular Weight:155.1546
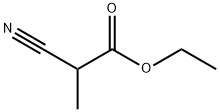
1572-99-2
162 suppliers
$14.00/1g
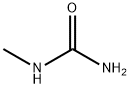
598-50-5
274 suppliers
$6.00/25g

1609259-60-0
11 suppliers
inquiry
Yield:1609259-60-0 80%
Reaction Conditions:
with hydrogenchloride;sodium methylate in methanol; for 18 h;Reflux;
Steps:
8.1 Step 1. Synthesis of6-amino-i ,5-dimethylpyrimidine-2,4(1H,3H)-dione,hydrochloride salt (C33)
Step 1.
Synthesis of 6-amino-1,5-dimethylpyrimidine-2,4(1H,3H)-dione, hydrochloride salt (C33)
A solution of sodium methoxide in methanol (4.4 M, 27 mL, 119 mmol) was added to a solution of ethyl 2-cyanopropanoate (95%, 13.2 mL, 99.6 mmol) and 1-methylurea (98%, 8.26 g, 109 mmol) in methanol (75 mL), and the reaction mixture was heated at reflux for 18 hours, then cooled to room temperature.
After removal of solvent in vacuo, the residue was repeatedly evaporated under reduced pressure with acetonitrile (3*50 mL), then partitioned between acetonitrile (100 mL) and water (100 mL).
Aqueous 6 M hydrochloric acid was slowly added until the pH had reached approximately 2; the resulting mixture was stirred for one hour.
The precipitate was collected via filtration and washed with tert-butyl methyl ether, affording the product as a white solid. Yield: 15.2 g, 79.3 mmol, 80%. LCMS m/z 156.1 [M+H+].
1H NMR (400 MHz, DMSO-d6) δ 10.38 (br s, 1H), 6.39 (s, 2H), 3.22 (s, 3H), 1.67 (s, 3H).
References:
US2014/128374,2014,A1 Location in patent:Paragraph 0372; 0373