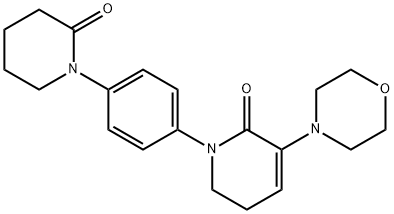
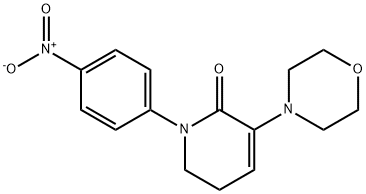
3-Morpholino-1-(4-(2-oxopiperidin-1-yl)phenyl)-5,6-dihydropyridin-2(1H)-one synthesis
- Product Name:3-Morpholino-1-(4-(2-oxopiperidin-1-yl)phenyl)-5,6-dihydropyridin-2(1H)-one
- CAS Number:545445-44-1
- Molecular formula:C20H25N3O3
- Molecular Weight:355.43
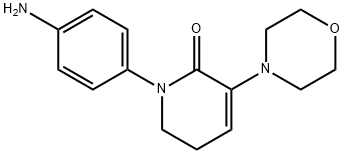
3-Morpholino-1-(4-(2-oxopiperidin-1-yl)phenyl)-5,6-dihydropyridin-2(1H)-one can be used as pharmaceutical intermediates and is synthesized with 5-Chlorovaleroyl chloride and 1-(4-aminophenyl)-3-(morpholin-4-yl)-5,6-dihydropyridin-2(1H)-one.
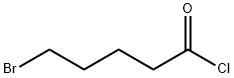
4509-90-4
233 suppliers
$15.00/1g
1267610-26-3
248 suppliers
$40.00/5g

545445-44-1
453 suppliers
$7.00/1g
Yield:545445-44-1 76%
Reaction Conditions:
Stage #1:5-bromovaleroyl chloride;1-(4-aminophenyl)-3-(morpholin-4-yl)-5,6-dihydropyridin-2(1H)-one with N-ethyl-N,N-diisopropylamine in tetrahydrofuran at 0 - 40; for 1 h;
Stage #2: with sodium hydride in ethyl acetate;tert-butyl alcohol at 10 - 40; for 3 h;Solvent;
Steps:
2 Preparation of Embodiment 4:3-morpholin-1 - [4 - (2-oxo-piperidinyl) phenyl] - 5,6-dihydro-pyridine -2 (1H)-one
General procedure: Room temperature, Compound 7 (5.8 g, 0.322 mmol), triethylamine 10. 6ml, tetrahydrofuran 176ml added to the reaction flask, stirring, cooling to 0 ° C, 5-chloropentanoyl chloride (5. 9g) Reaction lh; 0 ° C conditions, Sodium hydride (2 g, 0.083 mol) was added, Warm to Room temperature reaction 3h, The organic phase was washed with saturated brine once, dried over anhydrous magnesium sulfate, filtered and the solvent was evaporated to give a pale yellow solid. 15.3 g, nil Water ethanol recrystallization was a white solid, after drying 8. 2g, 0.023mol. Yield 72%, m.p. 202-204 ° C. HPLC: 99. 47% (instrument: Waters high performance liquid chromatography, column: C18, column length: 25 cm, mobile phase: water: Flow rate: l.Oml / min, wavelength: 254nm, column temperature: 30 ° C, injection volume: 10μ1) 1st step acidylated, cyclization reaction feeding compound 3 (5.35g, 0 . 06mol), diisopropylethylamine (18 ml), 5-bromo toluene (15g, 0.07mol), tert- butylacohol armor (16.8g, 0 . 15mol), ethyl acetate as solvent, getting white solid, heavy after drying 8.5 g. The yield is 76%, melting point 99-100°C. The embodiment of the remaining step is repeated 1 operation, prepare compound 2. The embodiment of the identification data for the 1 same in.
References:
Shanghai Institute of Pharmaceutical Industry;China State Institute of Pharmaceutical Industry;Huo, Shaowei;Guo, Yekun;Zhong, Jingfen;Shi, Huilin CN103626689, 2016, B Location in patent:Paragraph 0033; 0054-0056; 0059
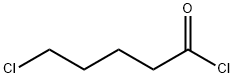
1575-61-7
356 suppliers
$14.00/1g

1267610-26-3
248 suppliers
$40.00/5g

545445-44-1
453 suppliers
$7.00/1g
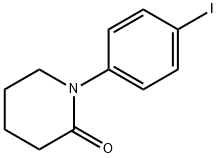
385425-15-0
141 suppliers
$40.00/500mg
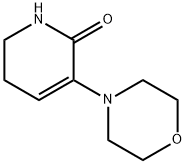
545445-40-7
74 suppliers
$26.00/100mg

545445-44-1
453 suppliers
$7.00/1g
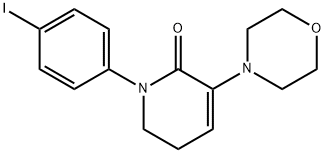
473927-69-4
105 suppliers
$201.00/250mg
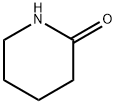
675-20-7
350 suppliers
$5.00/1g

545445-44-1
453 suppliers
$7.00/1g
503615-03-0
333 suppliers
$31.00/1g

545445-44-1
453 suppliers
$7.00/1g