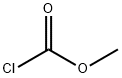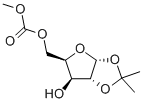
5-O-CARBOMETHOXY-1,2-O-ISOPROPYLIDENE-D-XYLOFURANOSE synthesis
- Product Name:5-O-CARBOMETHOXY-1,2-O-ISOPROPYLIDENE-D-XYLOFURANOSE
- CAS Number:5432-33-7
- Molecular formula:C10H16O7
- Molecular Weight:248.23
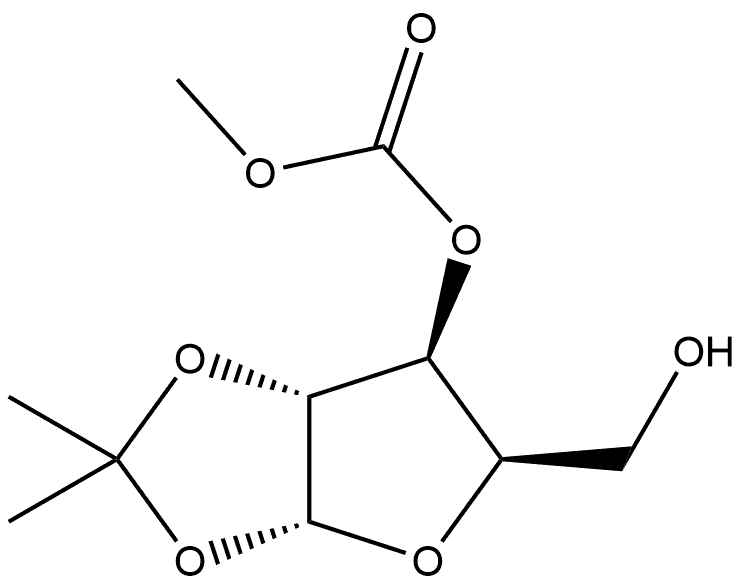
1388092-19-0
0 suppliers
inquiry

5432-33-7
28 suppliers
$68.00/1g
Yield:5432-33-7 95%
Reaction Conditions:
with aluminum oxide in ethanol;chloroform;Column chromatography;
Steps:
4.7.1. 1,2-O-Isopropylidene-3-O-methylcarbonate-α-d-xylofuranoside (3d)
For the reaction was used 80.0 mg (0.22 mmol) of 5-O-tert-butyldimethylsilyl-1,2-O-isopropylidene-3-O-methylcarbonate-α-d-xylofuranoside 1b, 2.00 mg (0.011 mmol) of CuCl2·2H2O and 2.2 mL of acetone/H2O (95/5). The reaction led to a formation of 3d (55.0 mg, 100%). Purification by neutral alumina column chromatography of 40 mg (gradient from 98/2 to 95/5, v/v, CHCl3/EtOH) led to an isolation of 38.0 mg of 1,2-O-isopropylidene-5-O-methylcarbonate-α-d-xylofuranoside 3a.
References:
Dvorakova, Marcela;Pribylova, Marie;Pohl, Radek;Migaud, Marie E.;Vanek, Tomas [Tetrahedron,2012,vol. 68,# 33,p. 6701 - 6711] Location in patent:experimental part
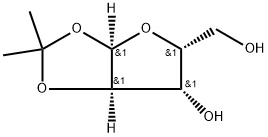
20031-21-4
260 suppliers
$6.00/1g

5432-33-7
28 suppliers
$68.00/1g
![α-D-Xylofuranose, 5-O-[(1,1-dimethylethyl)dimethylsilyl]-1,2-O-(1-methylethylidene)-, 3-(methyl carbonate)](/CAS/20210305/GIF/1388091-75-5.gif)