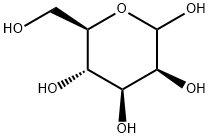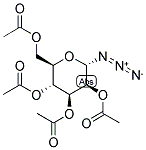
2,3,4,6-TETRA-O-ACETYL-ALPHA-D-MANNOPYRANOSYL AZIDE synthesis
- Product Name:2,3,4,6-TETRA-O-ACETYL-ALPHA-D-MANNOPYRANOSYL AZIDE
- CAS Number:53784-29-5
- Molecular formula:C14H19N3O9
- Molecular Weight:373.32
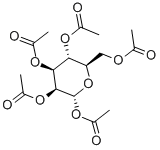
4163-65-9
194 suppliers
$15.00/1g

53784-29-5
49 suppliers
inquiry
Yield:53784-29-5 100%
Reaction Conditions:
with trimethylsilylazide;tin(IV) chloride in dichloromethane;Inert atmosphere;
Steps:
20 4.3 General procedure for glucopyranosyl azides (Procedure A)11
General procedure: To a solution of the O-acetyl monosaccharide in dry CH2Cl2 (4mL/mmol), TMS-N3 (2.5equiv) and SnCl4 (0.5equiv) were added under nitrogen. The reaction was stirred until completion (TLC). The mixture was then diluted with CH2Cl2, and an equal volume of saturated NaHCO3 solution was added and left for 30min under stirring. The mixture was then extracted with CH2Cl2, dried over anhydrous Na2SO4, and evaporated in vacuum. The product was purified by flash column chromatography. 4.20
2,3,4,6-Tetra-O-acetyl-α-d-mannopyranosyl azide 6
30
To a solution of 1,2,3,4,6-penta-O-acetyl-α-d-mannopyranose (500 mg, 1.28 mmol) in 5 mL of dry CH2Cl2, TMS-N3 (440 μL, 3.2 mmol) and SnCl4 (115 μL, 0.64 mmol) were added following Procedure A.
The reaction product (480 mg, quant.) was used without purification. TLC Rf = 0.77 (n-hexane/EtOAc, 7:3); NMR data of this compound are consistent with those reported in Ref.
30
.
References:
Tejera, Sara;Dorta, Rosa L.;Vázquez, Jesús T. [Tetrahedron Asymmetry,2016,vol. 27,# 17-18,p. 896 - 909]
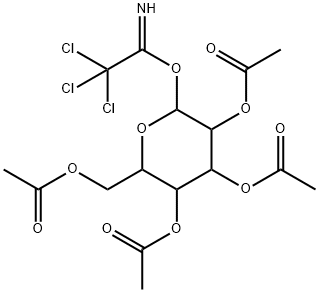
121238-27-5
51 suppliers
$80.00/200mg

53784-29-5
49 suppliers
inquiry
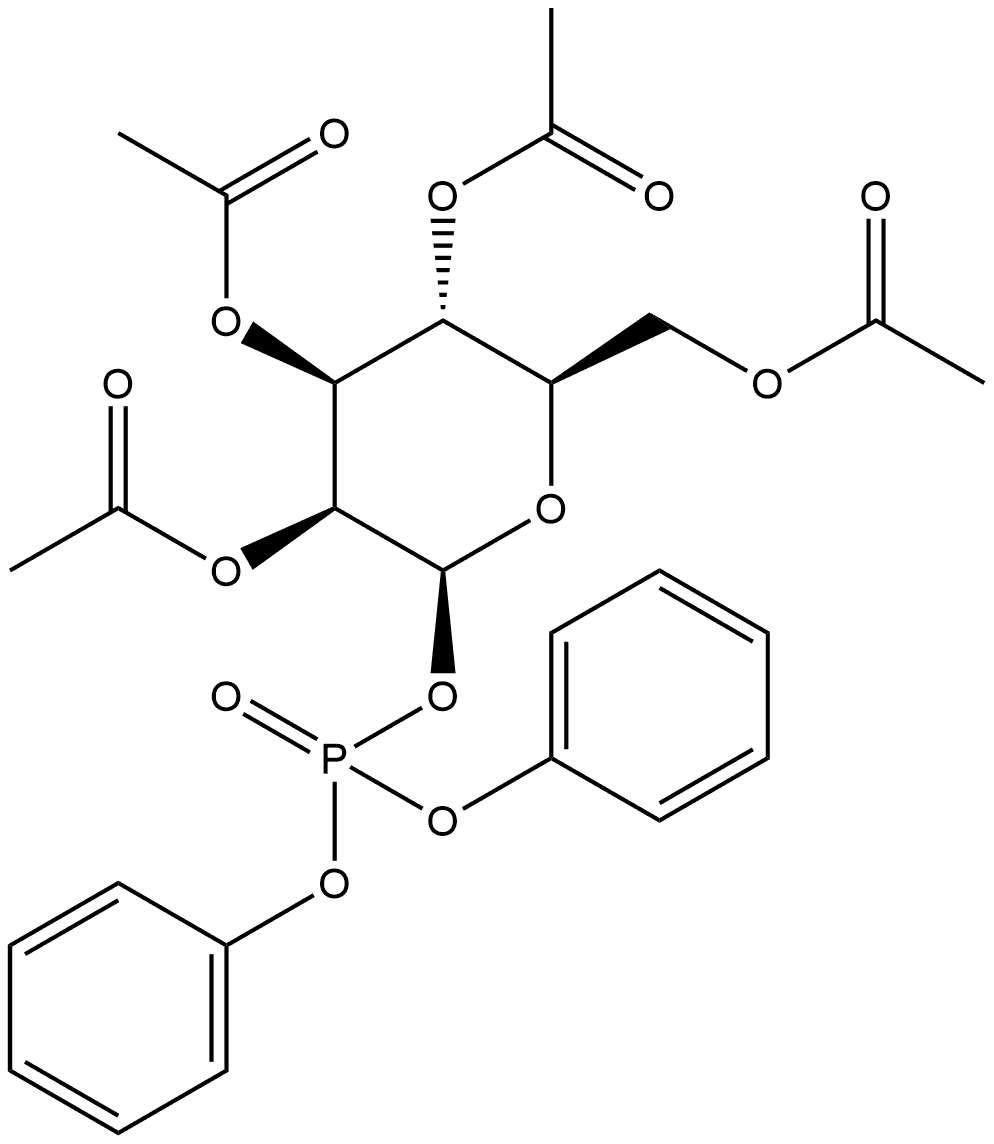
139710-25-1
0 suppliers
inquiry

53784-29-5
49 suppliers
inquiry
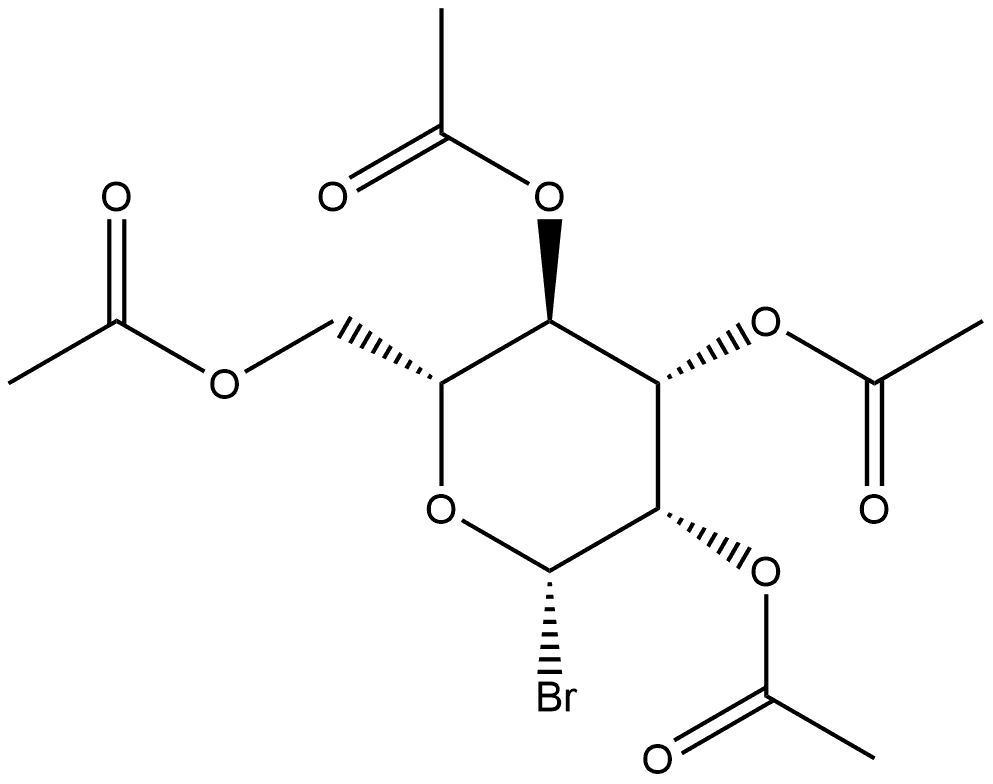
60619-58-1
0 suppliers
inquiry

53784-29-5
49 suppliers
inquiry