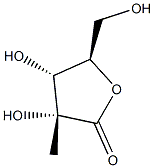
(3R,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl)-3-methyldihydrofuran-2(3H)-one synthesis
- Product Name:(3R,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl)-3-methyldihydrofuran-2(3H)-one
- CAS Number:53008-90-5
- Molecular formula:C6H10O5
- Molecular Weight:162.14

93635-76-8
143 suppliers
$10.00/5g

53008-90-5
3 suppliers
inquiry
Yield:53008-90-5 87.8%
Reaction Conditions:
in ethanol at 25 - 79; for 18 h;Large scale;
Steps:
12.A.1 Example 12. Large-Scale Manufacture of Compound 1, Compound 1-A, and Compound 2: Part A: Synthesis of Compound 2:
Step 1: Preparation of Compound 2-36: To a 50L four-necked double glass flask equipped with mechanical stirrer, addition funnel, condenser and thermometer, ethanol (24 Kg) was charged. Compound 2-35 (6 Kg, 24.17 mol., 1 eq) was charged in one portion at 10°C to afford a suspension. The mixture was warmed to 25°C for 20 minutes to afford a clear solution. Hydrochloride acid (36%, 6.1 Kg, 60.42 mol., 2.5 eq) was charged dropwise in 2 hours while the internal temperature reached to 32°C from 25°C to afford a light-yellow clear solution. The reaction mixture was heated to 79±2°C for 16 hours while maintaining the gentle reflux at which point TLC (DCM:MeOH=10: l, 1% aqueous KMnCh as the chromogenic reagent) showed the Compound 2-30 was consumed completely. The mixture was cooled to 40-45°C. The reaction mixture was transferred to a rotary evaporator (50 L) and then concentrated under vacuum (less than 0.09MPa) while maintaining at 60±5°C (water bath) until no fraction flowed out through condensing apparatus to afford a brown rope liquid. Anhydrous ethanol (12 Kg) was charged and the evaporation was repeated. Additional anhydrous ethanol (12 Kg) was charged and the evaporation was repeated once more. The crude product (oil) was dissolved in ethyl acetate (12 Kg) at 50±5°C to afford a clear solution (no acetylation impurities were found) and transferred to the 50L reactor. Water (0.625 Kg, 34.72 mol., 1.5 eq) was added dropwise maintaining the internal temperature between 25±5°C during 1 hour. After another 0.5 hour with stirring, solid precipitated. The resulting suspension was stirred at 15±5°C for 16 hours. The solid was filtered and washed with ethyl acetate (3 Kg). The wet cake was dried at 45±5°C for 16 hours in air oven without vacuum. Compound 2-36 (3.821 Kg) was obtained in a yield of 87.8% as a white solid. 1HNMR (DMSO-de): 8 5.82-5.75 (m, 2H), 5.11-5.08 (bs, 1H), 4.02-3.95 (m, 2H), 3.74-3.71 (m, 1H), 3.53- 3.48 (m, 3H), 1.19 (s, 3H).
References:
WO2022/40473,2022,A1 Location in patent:Page/Page column 140-141
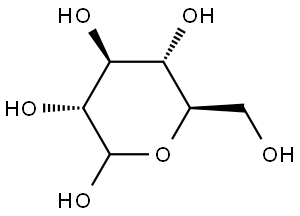
2280-44-6
12 suppliers
inquiry

53008-90-5
3 suppliers
inquiry
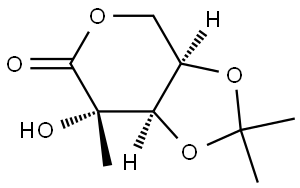
850359-46-5
0 suppliers
inquiry

53008-90-5
3 suppliers
inquiry
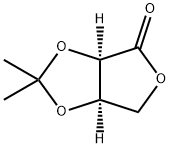
25581-41-3
113 suppliers
$24.00/250mg
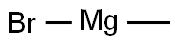
75-16-1
279 suppliers
$12.00/10ml

53008-90-5
3 suppliers
inquiry
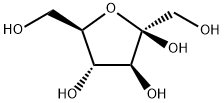
57-48-7
586 suppliers
$12.00/250G

53008-90-5
3 suppliers
inquiry