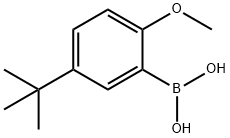
5-TERT-BUTYL-2-METHOXYBENZENEBORONIC ACID synthesis
- Product Name:5-TERT-BUTYL-2-METHOXYBENZENEBORONIC ACID
- CAS Number:128733-85-7
- Molecular formula:C11H17BO3
- Molecular Weight:208.06
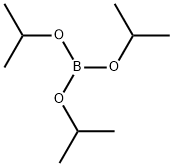
5419-55-6
376 suppliers
$12.00/5g
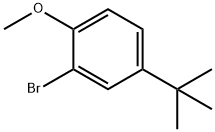
41280-65-3
36 suppliers
$130.00/500mg

128733-85-7
59 suppliers
$28.00/100mg
Yield: 74%
Reaction Conditions:
with n-butyllithium in tetrahydrofuran;hexane at -78 - 0; for 4.5 h;Inert atmosphere;
Steps:
5.2 (5-(tert-Butyl)-2-methoxyphenyl)boronic acid
n-Butyllithium in hexane (2.5 M, 18.1 mL, 45.24 mmol) was added dropwise to a solution of 2-bromo-4-(tert-butyl)-1-methoxybenzene (10.0 g, 41.13 mmol) and triisopropyl borate (11.39 mL, 49.35 mmol) in tetrahydrofuran (90 mL) at -78° C. under nitrogen.
The reaction mixture was stirred at -78° C. for 3 hours then allowed to warm slowly to 0° C. over 90 minutes.
The reaction was then quenched by the addition of water (90 mL) and the tetrahydrofuran removed under reduced pressure.
The resulting aqueous suspension was partitioned between diethyl ether (80 mL) and 1.0 M aqueous sodium hydroxide solution (100 mL).
The aqueous phase was separated, cooled to 0° C. then acidified to pH 1 by the addition of concentrated hydrochloric acid.
The resulting white suspension was stood at 0° C. for 15 minutes then filtered washing the solid product with water and cold hexane to give (5-(tert-butyl)-2-methoxyphenyl)boronic acid (6.349 g, 74% yield) as a white crystalline solid.
HPLC/MS Rt=5.04 min, m/z 209.1 (M+H+).
References:
Jacobus Pharmaceutical Company, Inc.;Heffernan, Gavin David;Jacobus, David Penman;Saionz, Kurt William;Schiehser, Guy Alan;Shieh, Hong-Ming;Zhao, Wenyi US2014/135320, 2014, A1 Location in patent:Paragraph 0241; 0242
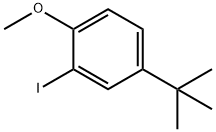
75060-39-8
1 suppliers
inquiry

128733-85-7
59 suppliers
$28.00/100mg
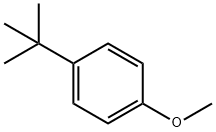
5396-38-3
136 suppliers
$10.00/1g

128733-85-7
59 suppliers
$28.00/100mg
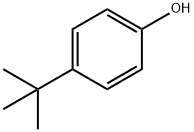
98-54-4
414 suppliers
$15.00/25g

128733-85-7
59 suppliers
$28.00/100mg
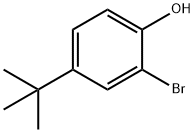
2198-66-5
182 suppliers
$5.00/1g

128733-85-7
59 suppliers
$28.00/100mg