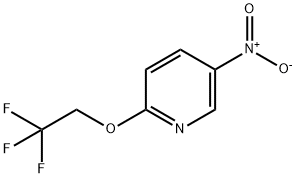
5-NITRO-2-(2,2,2-TRIFLUOROETHOXY)PYRIDINE synthesis
- Product Name:5-NITRO-2-(2,2,2-TRIFLUOROETHOXY)PYRIDINE
- CAS Number:72617-81-3
- Molecular formula:C7H5F3N2O3
- Molecular Weight:222.12
Yield:72617-81-3 93%
Reaction Conditions:
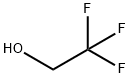
Stage #1: 2,2,2-trifluoroethanolwith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0; for 1.33333 h;
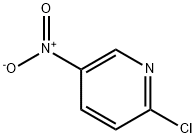
Stage #2: 2-chloro-5-nitropyridine in N,N-dimethyl-formamide;mineral oil at 0 - 20; for 2.5 h;
Steps:
108.1
Example 108(5a,8a)-8-Hydroxy-8-isopropyl-2-(6-(2,2,2-trifiuoroethoxy)pyridin-3-yl)- spiro [4.5] decan- 1 -oneStep 1: 5-Nitro-2-(21212-trifluoro-ethoxy)-pyridineIn a 250 mL four-necked flask, sodiumhydride (60 % in mineral oil, 4.16 g) was combined with DMF (60mL). The suspension was cooled to 0°C and 2,2,2-trifluoroethanol (10.4 g, [CAS Reg. No. 75-89-8]) was added dropwise over a period of 20 minutes to the cold suspension. The mixture was stirred for 1 hour at 0°C. Then, a solution of 2-chloro-5- nitropyridine (15 g, [CAS Reg. No. 4548-45-2]) in DMF (70mL) was added dropwise over a period of 20 minutes to the cold reaction mixture. Stirring was continued for 10 minutes at 0°C and for 2 hours at r.t.. The reaction mixture was poured into ice/water and was then extracted two times with ethyl acetate. The combined organic layers were washed with brine, dried over Na2S04, and filtered. The solvent was evaporated and the residue was purified by flash chromatography (silica gel, gradient of heptane in ethyl acetate) to give the title compound as a yellow solid (20.52g, 93%). MS (EI, m/e): 222.0 [M+].
References:
WO2011/45292,2011,A1 Location in patent:Page/Page column 175-176