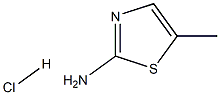
5-Methylthiazol-2-amine hydrochloride synthesis
- Product Name:5-Methylthiazol-2-amine hydrochloride
- CAS Number:6593-86-8
- Molecular formula:C4H7ClN2S
- Molecular Weight:150.6298
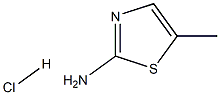
6593-86-8
4 suppliers
inquiry
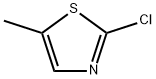
33342-65-3
136 suppliers
$30.00/250mg
Yield:33342-65-3 81%
Reaction Conditions:
with hydrogenchloride;sodium nitrite in water;
Steps:
2 Synthesis of 2-chloro-5-chloromethylthiazole
EXAMPLE 2 Synthesis of 2-chloro-5-chloromethylthiazole In a 200 ml volume three necked flask equipped with a stirrer, a dropping funnel and a thermometer, 14 g of 2-amino5-methylthiazole hydrochloride (0.093 mol), 25 ml of 36% hydrochloric acid and 20 ml of water were place and cooled to -5° C. To the mixture, 6.8 g of sodium nitrite (0.0986 mol) dissolved in 10 ml of water was gradually added dropwise at 0° C. or lower. The reaction mixture was further caused to react for one hour at 0° C. or lower to give the diazonium base. The reaction mixture was heated to 40° C. for three hours and extracted with three 40 ml portions of chloroform to give a chloroform solution containing 2-chloro-5-methylthiazole. The chloroform was removed by atmospheric distillation and remained fraction was distilled under reduced pressure to isolate 10.1 g of 2-chloro-5-methylthiazole (0.0756 mol) with a yield of 81%. The resulting 2-chloro-5-methylthiazole was dissolved in 20 ml of chloroform and placed in a 100 ml volume three necked flask equipped with a stirrer, a high pressure mercury lamp and a thermometer.
References:
US5811555,1998,A