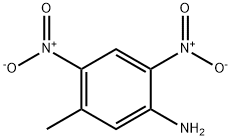
5-Methyl-2,4-dinitrobenzenamine synthesis
- Product Name:5-Methyl-2,4-dinitrobenzenamine
- CAS Number:5267-27-6
- Molecular formula:C7H7N3O4
- Molecular Weight:197.15
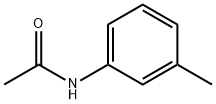
537-92-8
116 suppliers
$10.00/1g

5267-27-6
4 suppliers
inquiry
Yield:5267-27-6 37%
Reaction Conditions:
Stage #1: N-(m-tolyl)acetamidewith sulfuric acid;HNO3 at 2 - 20; for 4 h;Inert atmosphere;
Stage #2: with sulfuric acid at 80; for 4 h;Inert atmosphere;
Steps:
37 4.37. 5-Methyl-2,4-dinitroaniline (60)
Nitric acid (29.5 ml) was added in several portions in sulfuric acid (178 ml) at 2-5 °C. Then N-(meta-tolyl)acetamide (50 g, 0.335 mol) was added and the reaction mixture was warmed to RT.
After 4 h, the reaction mixture was poured into ice water (2 L) and an orange precipitate was formed.
The resulting suspension was stirred overnight at RT, filtered and the precipitate obtained was washed with water and dried.
The collected solid was solved in sulfuric acid 50% (350 ml) and stirred 4 h at 80 °C.
The reaction mixture was cooled down to RT, water (1 L) was added and the suspension obtained was stirred overnight at RT. The precipitate obtained was filtered, and dried.
The solid obtained was crystallized in ethyl acetate and cyclohexane to give 23.4 g 5-methyl-2,4-dinitro-aniline (60) as a yellow solid (37%).
1H NMR (DMSO-d6, 400 MHz): 8.78 (s, 1H); 8.15 (br s, 2H); 6.9 (s, 1H); 2.5 (s, 3H). MS (electrospray): m/z = 198 [M+1].
References:
Jeanguenat, André;Durieux, Patricia;Edmunds, Andrew J.F.;Hall, Roger G.;Hughes, Dave;Loiseleur, Olivier;Pabba, Jagadish;Stoller, André;Trah, Stephan;Wenger, Jean;Dutton, Anna;Crossthwaite, Andrew [Bioorganic and Medicinal Chemistry,2016,vol. 24,# 3,p. 403 - 427]
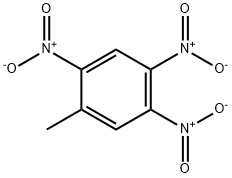
610-25-3
0 suppliers
inquiry

5267-27-6
4 suppliers
inquiry
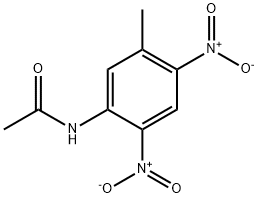
103752-65-4
0 suppliers
inquiry

5267-27-6
4 suppliers
inquiry
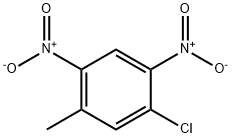
51676-74-5
62 suppliers
$10.00/250mg

5267-27-6
4 suppliers
inquiry
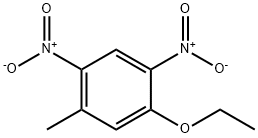
98953-57-2
0 suppliers
inquiry

5267-27-6
4 suppliers
inquiry