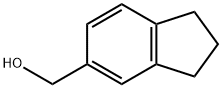
5-HYDROXYMETHYLINDANE synthesis
- Product Name:5-HYDROXYMETHYLINDANE
- CAS Number:51632-06-5
- Molecular formula:C10H12O
- Molecular Weight:148.2
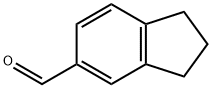
30084-91-4
106 suppliers
$74.00/250mg

51632-06-5
23 suppliers
inquiry
Yield:51632-06-5 96%
Reaction Conditions:
Stage #1: indan-5-carbaldehydewith sodium tetrahydroborate in ethanol at 0; for 0.5 h;
Stage #2: with hydrogenchloride in water; pH=2;
Steps:
10.2
To a stirred solution of Indan-5-carbaldehyde (2 g, 13.69 mmol) in EtOH (20 ml) at 0 0C was added sodium borohydride (676 mg, 17.80 mmol). The reaction was continued for 30 min. and monitored by thin layer chromatography. The reaction mixture was concentrated, acidified by addition of 2N HCl to pH 2. It was extracted with dichloromethane (2x 25 ml). The combined organic layer was washed with brine (25 ml), dried over anhydrous sodium sulfate and concentrated under vacuo to afford colorless oil (1.95 g , 96 %).
References:
WO2009/109999,2009,A1 Location in patent:Page/Page column 60
65898-38-6
58 suppliers
$49.00/100mg

51632-06-5
23 suppliers
inquiry
86031-43-8
17 suppliers
$45.00/10mg

51632-06-5
23 suppliers
inquiry
496-11-7
243 suppliers
$5.00/5g

51632-06-5
23 suppliers
inquiry

