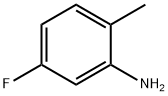
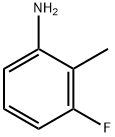
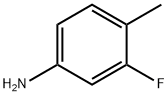
5-Fluoro-2-methylaniline synthesis
- Product Name:5-Fluoro-2-methylaniline
- CAS Number:367-29-3
- Molecular formula:C7H8FN
- Molecular Weight:125.14

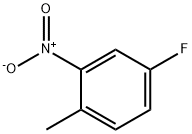
446-10-6
326 suppliers
$10.00/25 g

367-29-3
275 suppliers
$7.00/5g
Yield:367-29-3 70%
Reaction Conditions:
with hydrogenchloride;iron in ethanol;water at 0; for 12 h;Reflux;
Steps:
1.1 Step-i: Synthesis of 5-fluoro-2-methylaniline
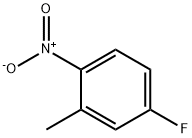
4-Fluoro-1-methyl-2-nitrobenzene (2.50 g, 16 mmol) was dissolved in ethanol (50 mL).To this solution, iron powder (4.50 g, 81 mmol) and 0.25 ml of HC1 were added at 0 °C and the reaction mixture was refluxed for 12h. After completion of reaction, reaction mixture was cooled to room temperature, diluted with ethyl acetate, filtered through Celite and washed with ethyl acetate. Filtrate was basified with sodium bicarbonate solution; organic layer was washed with water followed by brine solution. Organic layer was dried over anhydrous Na2SO4 andconcentrated under reduced pressure to obtain crude compound. The residue was purified by column chromatography (n-hexane/ EtOAc 1:1) to give the title compound (1.4 g, 70 %) as a light brown solid.‘H NMR (400 MHz, DMSO-d6): ? 6.87 (t, J = 7.6 Hz, 1H), 6.38-6.34 (m, 1H), 6.22-6.18 (m, 1H), 5.11 (bs, 2H), 1.99 (s, 3H). MS (ES) mle: 126 (M+1).
References:
AURIGENE DISCOVERY TECHNOLOGIES LIMITED;GUMMADI, Venkateshwar Rao;SAMAJDAR, Susanta;GUPTA, Ajay WO2015/104662, 2015, A1 Location in patent:Page/Page column 37
1422-53-3
334 suppliers
$5.00/5g

367-29-3
275 suppliers
$7.00/5g
452-73-3
350 suppliers
$6.00/25g

367-29-3
275 suppliers
$7.00/5g
446-33-3
253 suppliers
$17.00/5g

367-29-3
275 suppliers
$7.00/5g
372-19-0
322 suppliers
$10.00/1g
593-53-3
70 suppliers
inquiry
443-86-7
256 suppliers
$10.00/1g

367-29-3
275 suppliers
$7.00/5g
452-77-7
266 suppliers
$8.00/5g