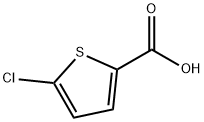
5-Chlorothiophene-2-carboxylic acid synthesis
- Product Name:5-Chlorothiophene-2-carboxylic acid
- CAS Number:24065-33-6
- Molecular formula:C5H3ClO2S
- Molecular Weight:162.59
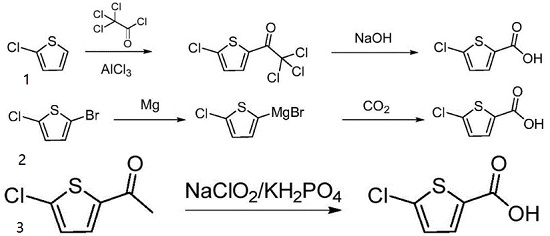
1. This method takes 2-chlorothiophene as an initial raw material, carrying out Friedel-crafts acylation with trichloroacetyl chloride under the action of aluminum trichloride to generate 2-trichloroacetyl-5-chlorothiophene, and then carrying out liquid alkali hydrolysis to obtain a target product.
2. This method reacts 5-chloro-2-bromothiophene with magnesium to generate a Grignard reagent, introducing carbon dioxide for inserting carbonyl and performing acid-base treatment to obtain a product.
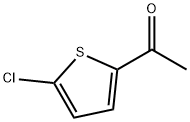
3. 5-chloro-2-acetylthiophene is used as a raw material and is oxidized by sodium chlorite and potassium dihydrogen phosphate system to obtain a target compound.
7283-96-7
273 suppliers
$8.00/5g

24065-33-6
764 suppliers
$6.00/5g
Yield:24065-33-6 98.8%
Reaction Conditions:
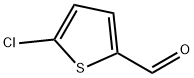
Stage #1: 5-Chloro-2-thiophenecarboxaldehydewith sodium hydroxide at -5 - 0; for 2 h;Large scale;
Stage #2: with chlorine at 15 - 30; for 8 h;Large scale;
Steps:
1-2 Example 1
The reaction solution was slowly dropped into an 11 kg (55 mol) 20% sodium hydroxide solution at 5 ° C while maintaining the internal temperature at -5 to 0 ° C, and the addition time was 2 hours. Control temperature 15~30 °C, After the addition is completed, 1.8 kg (25 mol) of chlorine gas is slowly introduced. Control temperature 15~30 °C, chlorine time 4 hours, After the completion of chlorine, the reaction was continued for 4 hours while maintaining the internal temperature at 15 to 30 ° C. TLC shows that the raw materials disappeared. HPLC showed the purity of the target product 5-chlorothiophene-2-carboxylic acid to be 92%. Stop the reaction, Cool down to 5~10 °C, Quenched by adding 10% aqueous sodium sulfite solution until the starch potassium iodide test paper does not change color. Add 5kg of dichloromethane, After stirring for 30 minutes, it was allowed to stand, and liquid was separated, and the obtained organic layer was packed in a bucket to be applied. 3.65kg (30mol) of 30% hydrochloric acid was added dropwise to the water layer to adjust the pH to 1~2. A large amount of white solid precipitated and was suction filtered. The wet weight of the filter cake was 3.6 kg, and the purity of the liquid phase was 96%. The filter cake was transferred to a 20 L round bottom four-necked flask, and 10.8 kg of ethanol and 3.6 kg of water were added. The temperature is raised to reflux, the system is completely dissolved, and the temperature is kept for 1 hour. Naturally cool down to 30 degrees and continue to cool down to 10 degrees, suction filtration, The filter cake was rinsed with 500 g of ethanol/water (ethanol: water = 3:1). Drying under reduced pressure gave 3.1 kg of the objective product 5-chlorothiophene-2-carboxylic acid, which was 98.8%.
References:
CN108840854,2018,A Location in patent:Paragraph 0027; 0029; 0030; 0032
6310-09-4
203 suppliers
$9.00/5g

24065-33-6
764 suppliers
$6.00/5g
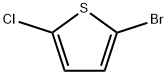
2873-18-9
180 suppliers
$8.00/1g
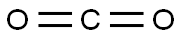
124-38-9
131 suppliers
$214.00/14L

24065-33-6
764 suppliers
$6.00/5g
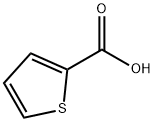
527-72-0
505 suppliers
$5.00/10g

24065-33-6
764 suppliers
$6.00/5g
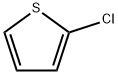
96-43-5
367 suppliers
$5.00/25g
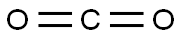
124-38-9
131 suppliers
$214.00/14L

24065-33-6
764 suppliers
$6.00/5g