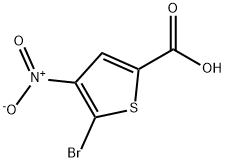
5-bromo-4-nitrothiophene-2-carboxylic acid synthesis
- Product Name:5-bromo-4-nitrothiophene-2-carboxylic acid
- CAS Number:89283-24-9
- Molecular formula:C5H2BrNO4S
- Molecular Weight:252.04
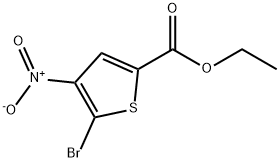
2160-52-3
22 suppliers
inquiry

89283-24-9
34 suppliers
inquiry
Yield:89283-24-9 83.35%
Reaction Conditions:
with lithium hydroxide monohydrate;potassium hydroxide in ethanol at 15; for 1 h;Inert atmosphere;
Steps:
1
A mixture of ethyl 5-bromo-4-nitro-thiophene-2-carboxylate (2 g, 7.14 mmol, 1 eq), (0166) KOH (801.24 mg, 14.28 mmol, 2eq) in EtOH (20 mL) and (10 mL) was degassed and purged with N? for 3 times, and then the mixture was stirred at 15 °C for 1 hr under Na atmosphere. The reaction was monitored by LCMS which showed complete consumption of reactant and defection of the desired mass peak. The reaction mixture was concentrated under reduced pressure. The reaction mixture was partitioned between HCi (50 ml, 1 N) and EtOAc (50 mL x 2). The organic phase was separated, washed with brine (50 mL), dried over anhydrous filtered and concentrated under reduced pressure to give a residue. The residue was purified by column chromatography ( Petroleum ether / Ethyl acetate ~ 50/1 to 0/1) to afford 5-bromo-4- nitro-thiophene-2 -carboxylic acid (1 .5 g, 5.95 mmol, 83.35% yieid) as a white solid.
References:
WO2022/76589,2022,A1 Location in patent:Page/Page column 76
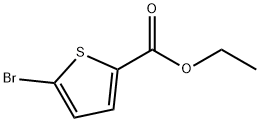
5751-83-7
150 suppliers
$6.00/1g

89283-24-9
34 suppliers
inquiry
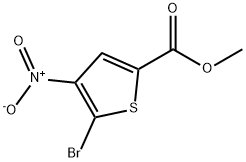
38239-32-6
70 suppliers
$22.00/100mg

89283-24-9
34 suppliers
inquiry
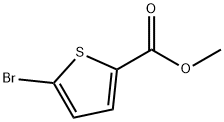
62224-19-5
150 suppliers
$11.00/1g

89283-24-9
34 suppliers
inquiry
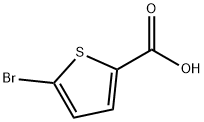
7311-63-9
339 suppliers
$5.00/250mg
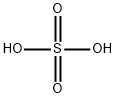
7664-93-9
6 suppliers
$22.69/1L
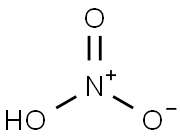
7697-37-2
8 suppliers
$13.00/25g

89283-24-9
34 suppliers
inquiry
2160-38-5
8 suppliers
inquiry