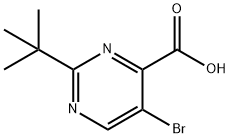
5-broMo-2-tert-butyl-pyriMidine-4-carboxylic acid synthesis
- Product Name:5-broMo-2-tert-butyl-pyriMidine-4-carboxylic acid
- CAS Number:59950-52-6
- Molecular formula:C9H11BrN2O2
- Molecular Weight:259.1
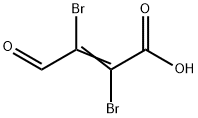
21577-50-4
20 suppliers
$75.00/2.5g

59950-52-6
13 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: pivalamidine hydrochloridewith sodium ethanolate in ethanol at 50;
Stage #2: mucobromic acid in ethanol at 55;
Stage #3: with hydrogenchloride;sodium ethanolatemore than 3 stages;
Steps:
C1.1
A 22% solution of sodium ethoxide in ethanol (53 mL, 165 mmol) was added dropwise to a magnetically stirred suspension of tert-butylcarbamidine hydrochloride (20.0 g, 146 mmol) in ethanol (100 mL). When the addition was complete, the yellow suspension was warmed to 50° C., the heating mantle was removed, and a solution of mucobromic acid (15.7 g, 61 mmol) in ethanol (50 mL) was added dropwise at a rate which did not allow the temperature to exceed 55° C. When this addition was complete, a 22% solution of sodium ethoxide in ethanol (32 mL, 98 mmol) was added dropwise, then the mixture was allowed to cool to room temperature. The suspension was filtered, the solids were rinsed with ethanol (2*20 mL), and the combined filtrates were concentrated in-vacuo. The residue thus obtained was stirred in 2 N aqueous HCl (30 mL). The resulting solids were collected by filtration, rinsed with ice-cold water (2*20 mL), and air dried to yield 12.1 g of 5-Bromo-2-tert-butyl-pyrimidine-4-carboxylic acid as a beige powder. MS (ES+)=259, 261 (M+H)+.
References:
US2005/54627,2005,A1 Location in patent:Page/Page column 38

21577-50-4
20 suppliers
$75.00/2.5g
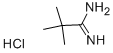
18202-73-8
126 suppliers
$7.00/250mg

59950-52-6
13 suppliers
inquiry