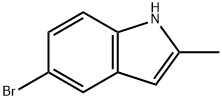
5-BROMO-2-METHYLINDOLE synthesis
- Product Name:5-BROMO-2-METHYLINDOLE
- CAS Number:1075-34-9
- Molecular formula:C9H8BrN
- Molecular Weight:210.07
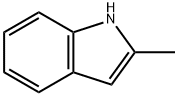
95-20-5
384 suppliers
$5.00/10g

1075-34-9
146 suppliers
$8.00/100mg
Yield: 75%
Reaction Conditions:
Stage #1:2-methyl-1H-indole with sulfuric acid;silver sulfate for 0.5 h;Cooling with ice;
Stage #2: with bromine at 20; for 4.5 h;
Steps:
75.1
Step 1. 5-Bromo-2-methyl-7H-indoleTo a solution of 2-methyl-iH-indole (5.0 g, 38.12 mmol) in sulfuric acid (80 mL) was added Ag2S04 (12.5 g, 40.06 mmol) with ice cooling, and the solution was stirred for 30 min. Then Br2 (6.4 g, 40.05 mmol) was added to the solution dropwise over 30 min. After the solution was stirred for 4 h at room temperature, the reaction was then quenched by the addition of water/ice (300 mL). The reaction mixture was extracted with dichloromethane (3 x 200 mL) and the organic layers combined, dried over anhydrous sodium sulfate and concentrated in vacuo to afford 5-bromo-2-methyl-7H-indole as a light brown solid (6 g, 75%).LC/MS (ES, m/z): [M+H]+ 211.0'H-NMR (300 MHz, CDC13): δ 11.23 (s, 1Η), 7.56 (s, 1Η), 7.21 (d, / = 8.7 Hz, 1H), 7.07 - 7.09 (m, 1H), 6.11 (s, 1H), 2.38 (s, 3H)
References:
BIOENERGENIX;MCCALL, John M.;ROMERO, Donna L.;KELLY, Robert C. WO2012/119046, 2012, A2 Location in patent:Page/Page column 161
20357-20-4
145 suppliers
$5.00/250mg
56904-86-0
0 suppliers
inquiry

1075-34-9
146 suppliers
$8.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1075-34-9
146 suppliers
$8.00/100mg
106-40-1
468 suppliers
$12.00/5g

1075-34-9
146 suppliers
$8.00/100mg
13061-96-6
298 suppliers
$6.00/1g
1240045-42-4
6 suppliers
inquiry

1075-34-9
146 suppliers
$8.00/100mg