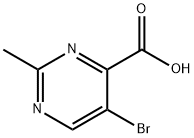
5-Bromo-2-methyl-4-pyrimidinecarboxylic acid synthesis
- Product Name:5-Bromo-2-methyl-4-pyrimidinecarboxylic acid
- CAS Number:100707-39-9
- Molecular formula:C6H5BrN2O2
- Molecular Weight:217.02
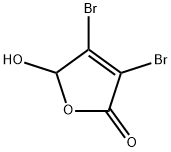
766-38-1
23 suppliers
$176.22/1g
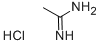
124-42-5
356 suppliers
$6.00/25g

100707-39-9
129 suppliers
$18.00/100mg
Yield:100707-39-9 42%
Reaction Conditions:
Stage #1: acetamidine hydrochloridewith sodium ethanolate in ethanol at 50;
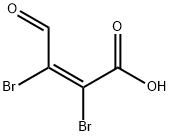
Stage #2: mucobromic acidwith sodium ethanolate in ethanol;
Steps:
42.42a
Step 42a: 5-Bromo-2-methylpyrimidine (Compound 0601-120)Sodium (356 mg, 15.5 mmol) was carefully added to ethanol (5.9 mL) to prepare sodium ethoxide solution in ethanol. The above freshly prepared sodium ethoxide in ethanol solution (3.5 mL) was added to a stirred suspension of acetamidine hydrochloride (0.91 g, 9.69 mmol). The mixture was warmed to 50 °C, then the heating bath was removed and a solution of mucobromic acid (1 g, 3.87 mmol) in ethanol was added dropwise at a rate which maintained a constant temperature, followed by a further sodium ethoxide in ethanol solution (2 mL). After cooling, the mixture was filtered and evaporated to a residue which was shaken vigorously with hydrochloric acid (2 M x 2.4 mL). The brown precipitate was filtered and washed with cold water, then freeze-dried to give 5-bromo-2- methylpyrimidine-4-carboxylic acid (350 mg, 42%) as a brown solid. LCMS: 218 [M+l]+, 1H NMR (400 MHz, DMSO-d6): δ 2.62 (s, 3H), 9.03 (s, 1H).
References:
WO2011/130628,2011,A1 Location in patent:Page/Page column 176
90994-06-2
5 suppliers
inquiry
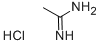
124-42-5
356 suppliers
$6.00/25g

100707-39-9
129 suppliers
$18.00/100mg
488-11-9
263 suppliers
$5.00/5g
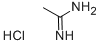
124-42-5
356 suppliers
$6.00/25g

100707-39-9
129 suppliers
$18.00/100mg