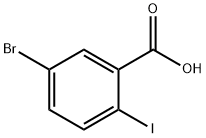
5-Bromo-2-iodobenzoic acid synthesis
- Product Name:5-Bromo-2-iodobenzoic acid
- CAS Number:21740-00-1
- Molecular formula:C7H4BrIO2
- Molecular Weight:326.91
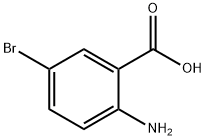
5794-88-7
462 suppliers
$6.00/10g

21740-00-1
321 suppliers
$8.00/5g
Yield:21740-00-1 87%
Reaction Conditions:
Stage #1:5-Bromo-2-aminobenzoic acid with hydrogenchloride;sodium nitrite in water at 0 - 40; for 3 h;
Stage #2: with sulfuric acid;potassium iodide in water at 90; for 1.33333 h;
Steps:
1 Example 1. Synthesis of 2-iodo-5-bromobenzoic acid (2)
2-Amino-5-bromobenzoic acid (2.14 g, 10 mmol), NaNO2 (0.828 g, 12 mmol) and NaOH (0.55 g,11 mmol) was dissolved in 40 ml of water, stirred, cooled to 0 ° C in an ice bath, and 12 ml of a 6 mol/L hydrochloric acid solution was added dropwise thereto, and the mixture was dropped over 2 hours. After the completion of the dropwise addition of hydrochloric acid, the reaction system was continued at 0 ° C for 1 h, then the reaction system was heated to 35-40 ° C, and a solution of KI [(KI 2.5 g, 15 mmol), H 2 SO 4 (0.6 ml) and water (5 ml) was slowly added. ], 20min added. The reaction system was then heated to 90 ° C for 1 h. After the reaction,The reaction system was slowly cooled to room temperature to precipitate a large amount of solids.Filtered, washed with water to give a crude yellow product. The crude product was recrystallized from 50% ethanol to give a pale yellow solid, 2.87g.The yield was 87%.
References:
Jiangsu Engineering Polytechnic College;Feng Chengliang;Yao Wenjin;Yan Bin CN109678686, 2019, A Location in patent:Paragraph 0053-0055
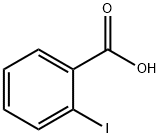
88-67-5
535 suppliers
$5.00/5g

21740-00-1
321 suppliers
$8.00/5g
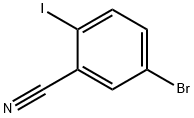
121554-10-7
129 suppliers
$34.00/1g

21740-00-1
321 suppliers
$8.00/5g
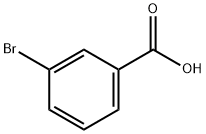
585-76-2
345 suppliers
$6.00/5g

21740-00-1
321 suppliers
$8.00/5g
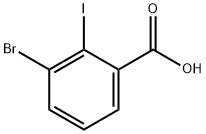
503821-94-1
67 suppliers
$42.00/250mg
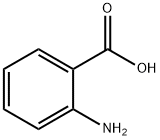
118-92-3
4 suppliers
$21.60/25g

21740-00-1
321 suppliers
$8.00/5g