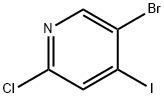
5-Bromo-2-chloro-4-iodopyridine synthesis
- Product Name:5-Bromo-2-chloro-4-iodopyridine
- CAS Number:401892-47-5
- Molecular formula:C5H2BrClIN
- Molecular Weight:318.34
53939-30-3
460 suppliers
$5.00/1g

401892-47-5
67 suppliers
$17.00/1g
Yield: 75.4%
Reaction Conditions:
Stage #1:5-bromo-2-chloropyridine with n-butyllithium;diisopropylamine in tetrahydrofuran at -78; for 1 h;
Stage #2: with iodine in tetrahydrofuran at 20; for 16 h;
Steps:
13.1 Step 1: 5-Bromo-2-chloro-4-iodopyridine
A stirring solution of DIPA (34 mL, 227.7 mmol, 1.1 eq) in dry THF (250 mL) was cooled to -78 °C and n-BuLi (85 mL, 207.9 mmol, 1.0 eq, 2.5 M in THF) was added dropwise. The resulting reaction mixture was stirred for 30 mi 5-Bromo- 2-chloropyridine (40 g, 207.9 mmol, 1 eq.) in dry THF (450 mL) was added dropwise and the reaction mixture was stirred at -78 °C for 1 h. A solution of iodine (55 g, 207.9 mmol, leq) in THF (250 mL) was added dropwise and the mixture was stirred for 16 h at RT. TLC analysis indicated formation of non-polar spot. The mixture was quenched with sodium thiosulfate solution (500 mL) and extracted with EtOAc (2 x 500 mL). The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure gave crude product which was recrystallized from ethanol (120 mL) to afford 5-bromo-2-chloro-4-iodopyridine (50 g, 75.4% yield) as an off white solid. LCMS:[M+Hjb 320.15.
References:
PROPELLON THERAPEUTICS INC.;AL-AWAR, Rima;ISAAC, Methvin;JOSEPH, Babu;LIU, Yong;MAMAI, Ahmed;PODA, Gennady;SUBRAMANIAN, Pandiaraju;UEHLING, David;WILSON, Brian;ZEPEDA-VELAZQUEZ, Carlos Armando WO2019/46944, 2019, A1 Location in patent:Paragraph 00161