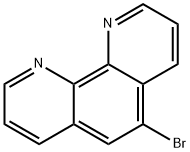
5-bromo-1,10-phenanthroline synthesis
- Product Name:5-bromo-1,10-phenanthroline
- CAS Number:40000-20-2
- Molecular formula:C12H7BrN2
- Molecular Weight:259.1
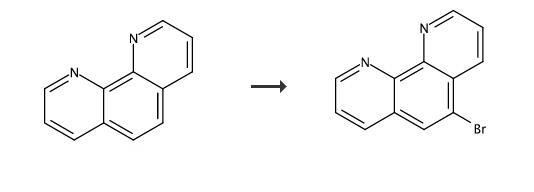
The compound was synthesized using a modification of the preparation described by Mlochowski.35 The yield of product is quite sensitive to the amount of bromine employed, the temperature, and the reaction time. Higher temperatures result in the formation of varying amounts of 5,6-dibromophenanthroline and 1,10-phenanthroline-5,6-dione. In our hands, the following preparation resulted in the maximum yield of product. A 3.6 g (20 mmol) sample of phenanthroline was placed in a heavy-walled glass reaction tube with a Teflon screw top fitted with a Viton O-ring. The reaction vessel was placed in an ice bath, and 12 mL of oleum (15%) and 0.60 mL (11.6 mmol) of bromine were added. The reaction tube was placed in a silicon oil bath, and the temperature was slowly raised to 135 °C. After 23 h, the reaction mixture was cooled to room temperature, poured over ice, and neutralized with NH4OH. The mixture was extracted with CHCl3. The extracts were stirred with charcoal and then dried over Na2SO4. The crude reaction mixture contained about 5% unreacted phenanthroline as judged by 1H NMR spectroscopy. The solid was recrystallized from hot diethyl ether with a minimum amount of CH2Cl2. Yield 4.67 g (90%).
53472-18-7
120 suppliers
$45.00/50mg

504-63-2
539 suppliers
$9.00/5g

40000-20-2
128 suppliers
$10.00/250mg
Yield:-
Reaction Conditions:
with 2,4,6-trimethyl-pyridine;palladium diacetate;trifluoroacetic acid at 150; under 760.051 Torr; for 12 h;
Steps:
1 Preparation of intermediate A-1
22.31 g (100 mmol) of 5-bromo-8-quinolinamide (Y), 7.61 g (100 mmol) of 1,3-propanediol, 1.12 g (5 mmol) of palladium acetate, 1.21g (5mmol) 2,4,6-trimethylpyridine, 1.14 g (10 mmol) of trifluoroacetic acid was heated to 150 ° C under an oxygen atmosphere (1 atm) for 12 hours. After completion of the reaction, it was filtered, and the filtrate was concentrated and purified by column chromatography to afford Intermediate A-1.
References:
CN108530443,2018,A Location in patent:Paragraph 0058; 0060-0062
66-71-7
385 suppliers
$6.00/5g

40000-20-2
128 suppliers
$10.00/250mg
57339-57-8
48 suppliers
$184.00/250mg

56-81-5
1662 suppliers
$5.00/25g

40000-20-2
128 suppliers
$10.00/250mg

53472-18-7
120 suppliers
$45.00/50mg

56-81-5
1662 suppliers
$5.00/25g

40000-20-2
128 suppliers
$10.00/250mg