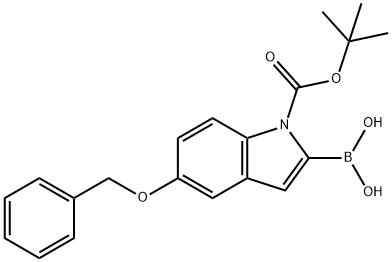
5-BENZYLOXY-1-BOC-INDOLE-2-BORONIC ACID synthesis
- Product Name:5-BENZYLOXY-1-BOC-INDOLE-2-BORONIC ACID
- CAS Number:850568-62-6
- Molecular formula:C20H22BNO5
- Molecular Weight:367.2
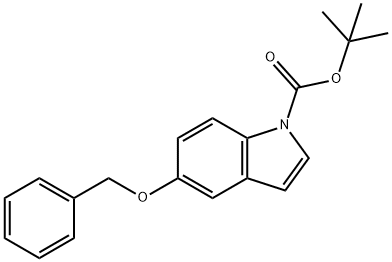
170147-29-2
39 suppliers
$14.00/250mg

850568-62-6
69 suppliers
$70.00/250MG
Yield:850568-62-6 100%
Reaction Conditions:
with Triisopropyl borate in tetrahydrofuran;n-heptane;ethylbenzene; for 2.41667 h;Cooling with ice;
Steps:
56.b
To a solution of 1,1 -dimethylethyl 5-[(phenylmethyl)oxy]-lH-indole-l -carboxylate(1.76 g, 4.8 mmol) in tetrahydrofuran (10 mL) was added triisopropyl borate (2.2 mL, 9.5 mmol). The solution was stirred in an ice-water bath under a nitrogen atmosphere
References:
WO2009/5998,2009,A1 Location in patent:Page/Page column 256-257
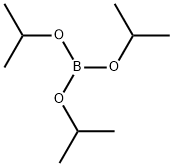
5419-55-6
376 suppliers
$12.00/5g

170147-29-2
39 suppliers
$14.00/250mg

850568-62-6
69 suppliers
$70.00/250MG
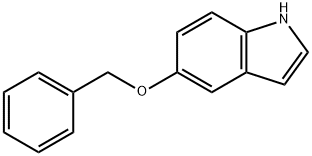
1215-59-4
335 suppliers
$10.00/1g

850568-62-6
69 suppliers
$70.00/250MG

24424-99-5
835 suppliers
$13.50/25G

850568-62-6
69 suppliers
$70.00/250MG