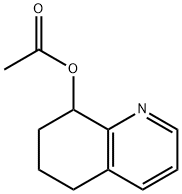
5,6,7,8-TETRAHYDRO-8-QUINOLINOL ACETATE synthesis
- Product Name:5,6,7,8-TETRAHYDRO-8-QUINOLINOL ACETATE
- CAS Number:14631-47-1
- Molecular formula:C11H13NO2
- Molecular Weight:191.23
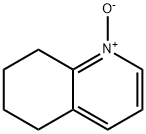
14631-48-2
35 suppliers
$75.00/500mg

14631-47-1
18 suppliers
$178.19/1g
Yield: 690 mg (3.6 mmol, 33%)
Reaction Conditions:
with acetic anhydride
Steps:
S.29.d Synthesis Example 29d
Synthesis Example 29d 8-Acetoxy-5,6,7,8-tetrahydroquinoline 5,6,7,8-Tetrahydroquinoline N-oxide (1.6 g, 11 mmol) was mixed with acetic anhydride (9.2 ml) and stirred at 90° C. for 7 hours. After removing the solvent by evaporation under a reduced pressure, the residue was neutralized with sodium hydroxide aqueous solution (1 N) and the reaction product was extracted with chloroform. The extract was washed with saturated brine and dried with anhydrous sodium sulfate, and then the solvent was removed by evaporation under a reduced pressure and the thus obtained material was separated and purified by a silica gel column chromatography to obtain 690 mg (3.6 mmol, 33%) of the title compound. 1H-NMR (CDCl3): δ 1.80-2.00 (2H, m), 2.01-2.20 (5H, m), 2.71-2.81 (1H, m), 2.83-2.91 (1H, m), 5.98 (1H, t, J=4.6 Hz), 7.16 (1H, dd, J=4.7 Hz, 7.8 Hz), 7.45 (1H, d), 8.49 (1H, d); MW 191.23 (C11H13NO2); Mass spectrum TSP m/z 192 (M+H)+.
References:
Meiji Seika Kaisha, Ltd. US6498251, 2002, B1
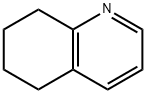
10500-57-9
288 suppliers
$6.00/5g

14631-47-1
18 suppliers
$178.19/1g

14631-48-2
35 suppliers
$75.00/500mg
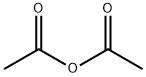
108-24-7
5 suppliers
$14.00/250ML

14631-47-1
18 suppliers
$178.19/1g