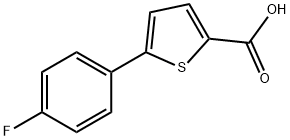
5-(4-Fluorophenyl)thiophene-2-carboxylic acid synthesis
- Product Name:5-(4-Fluorophenyl)thiophene-2-carboxylic acid
- CAS Number:115933-30-7
- Molecular formula:C11H7FO2S
- Molecular Weight:222.24
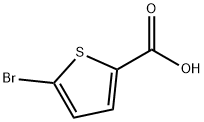
7311-63-9
338 suppliers
$5.00/250mg
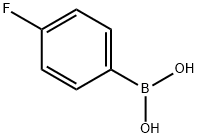
1765-93-1
557 suppliers
$9.00/1g

115933-30-7
71 suppliers
$45.00/50mg
Yield: 71%
Reaction Conditions:
with potassium phosphate;tetrakis(triphenylphosphine) palladium(0) in N,N-dimethyl-formamide at 105;Inert atmosphere;Suzuki Coupling;
Steps:
4.1.1 Synthesis of substituted 5-phenylfuran/thiophene-2-carboxylic acid (1a-1d) and (7a- 7d)
General procedure: 5-bromofuran-2-carboxylic acid/ 5-bromothiophene-2-carboxylic acid (0.0013mol), substituted boronic acid (0.0015mol), Pd(Ph3)4 (10mol %), were added in the a round bottom flask. K3PO4 (0.0026mol), and dry 11 DMF (5mL) were added and reaction was performed at 105°C under nitrogen atmosphere for 15-20h. After the completion of reaction, reaction mixture was quenched with cold water (15mL), acidified with HCl (0.5mL), followed by extraction with EtOAc (25×4mL). The combined EtOAc layer was washed with saturated sodium bicarbonate solution (10×2mL) to remove excess of HCl. The EtOAc layer, was filtered through celite bed over cotton, to remove Pd(Ph3)4, and concentrated under vacuum. The crude product was adsorbed and loaded on silica gel (100-200 mesh) column. The column was eluted with ethyl acetate (10-20%) in hexane to give desired compound in yield ranging from 43 to 71%.
References:
Kumar, Gautam;Krishna, Vagolu Siva;Sriram, Dharmarajan;Jachak, Sanjay M. [European Journal of Medicinal Chemistry,2018,vol. 156,p. 871 - 884]
333793-04-7
9 suppliers
inquiry

115933-30-7
71 suppliers
$45.00/50mg
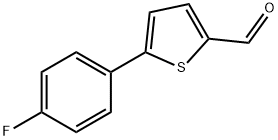
249504-38-9
35 suppliers
$155.00/50mg

115933-30-7
71 suppliers
$45.00/50mg
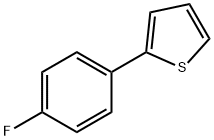
58861-48-6
355 suppliers
$8.00/1g
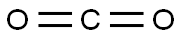
124-38-9
129 suppliers
$175.00/23402

115933-30-7
71 suppliers
$45.00/50mg
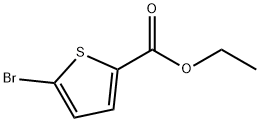
5751-83-7
150 suppliers
$6.00/1g

115933-30-7
71 suppliers
$45.00/50mg