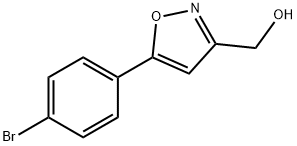
(5-(4-BROMOPHENYL)ISOXAZOL-3-YL)METHANOL synthesis
- Product Name:(5-(4-BROMOPHENYL)ISOXAZOL-3-YL)METHANOL
- CAS Number:640291-96-9
- Molecular formula:C10H8BrNO2
- Molecular Weight:254.08
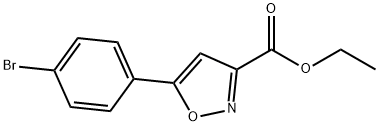
33277-15-5
33 suppliers
$83.00/100mg

640291-96-9
7 suppliers
inquiry
Yield:640291-96-9 70%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0; for 1 h;
Steps:
11.1
Ethyl 5-(4-bromophenyl)isoxazole-3-carboxylate (2.0 g, 6.75 mmol) synthesized in Step 2 of Preparation Example 10 was dissolved in tetrahydrofuran (40 mL), and 2 equivalents of lithium aluminum hydride (LAH) were gradually added to the solution at 0° C. After reaction for 1 hour, 1N-NaOH and 1N-HCl were added to terminate the reaction, followed by extraction with ethyl acetate (100 mL). The organic layer was washed with water (100 mL×2) and brine (50 mL). The organic layer was separated, dried over anhydrous magnesium sulfate, and filtered under reduced pressure to remove ethyl acetate. The residue was purified by silica gel column chromatography using ethyl acetate and hexane as a developing solvent, thus affording the title compound 5-(4-bromophenyl)isoxazol-3-yl)methanol(12f). Yield: 70%.1H NMR (CDCl3, 400 MHz): 7.60 (m, 4H), 6.57 (s, 1H), and 4.79 (s, 2H).
References:
US2010/63041,2010,A1 Location in patent:Page/Page column 33; 34
40155-54-2
18 suppliers
$55.00/250mg

640291-96-9
7 suppliers
inquiry
99-90-1
471 suppliers
$6.00/25g

640291-96-9
7 suppliers
inquiry